Before we get started, let me be clear that any information contained here, although to be best of my knowledge accurate, in in no way intended to be a substitute for advice and care from licensed medical professionals. OK, disclaimer stated.
Aspirin is one of the first synthetic drugs, and is still in wide use after over 100 years. It was first marketed by Bayer in 1899, and sales are still strong despite competition from drugs like acetaminophen, ibuprofen, and naproxin sodium. Bayer has in the past week or two come out with a new advert about its new “quick acting” aspirin.
This material is “quick acting” because the particle size is much smaller than that of regular aspirin. Since aspirin is only slowly soluble in water, the greater surface area for the same mass does speed up absorption.
Aspirin, more properly called acetylsalicylic acid or ASA, has been known since the mid 1800s, but only used as a drug since 1899 when Bayer started marketing it. The term aspirin was originally a trademark of Bayer’s but in many countries, including the US, Bayer has lost the right to the trademark. Just as an aside, at about the same time Bayer was also marketing diacetylmorphine, with the trademark name of heroin.
Here is the chemical structure for aspirin:
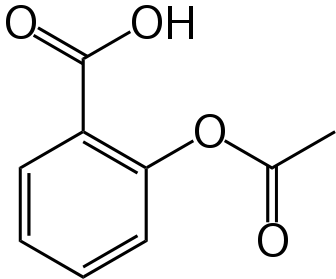
Note that it is a rather simple molecule as drugs go. It is produced by “capping” the free-OH group of salicylic acid with an acetyl group. It turns out that salicylic acid is also an effective drug, but with the -OH group uncapped is much more irritating to the GI track than is aspirin. Here is the chemical structure for salicylic acid:
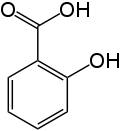
Salicylic acid is available large quantities in a high state of purity and very cheaply, and capping the hydroxy group is easy and cheap too. As a matter of fact, when I was a teaching assistant in graduate school that synthesis was a standard laboratory procedure in Organic Chemistry laboratory.
Actually, salicylates have been used since antiquity to treat fever and pain, but no one knew it. It turns out that there is a material in the bark of willow trees (genus name Salix) called salicin that is a relative of salicylic acid. Here is the chemical structure for salicin:
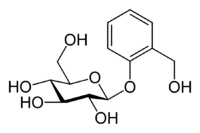
Do not worry about that big group on the left. It is just a glucose (sugar) molecule, which often is bonded to other materials in the plant kingdom.
It is, through a rather complex process, metabolized to salicylic acid when consumed, so willow bark tea actually was quite useful back in the ancient world. It is still used as a folk remedy in many areas and still works well.
Back in 1899 when aspirin was first marketed the mechanisms for its actions were completely unknown. I use the plural because aspirin has many different actions and many different mechanisms for those actions. About all that they knew at the time was that it was much better tolerated than the salicylate then in use, sodium salicylate, and just as effective at reducing fever, inflammation, and pain. They had no idea why it worked or of the many other actions other than these that it has.
Most people think of aspirin as a pain reliever and fever reducer. It is outstanding at those activities, being more effective than most other drugs (except for ibuprofen and narcotics for pain) for these purposes. Aspirin is not so good good for sudden pains, but rather for “dull” aches and throbs, like headache and joint pain. Interestingly, aspirin is just about as good as some of the newer, prescription only migraine drugs, and is much cheaper. Normally, a full gram is used for migraine while only about half that for other pain.
Aspirin has a multitude of actions, and not all have to do with pain. It turns out that the inflammatory process is mediated mainly by inhibition of cyclooxygenase enzymes (there are at least two subtypes, PTGS1 and PTGS2). Aspirin donates its acetyl group to the receptor site of the enzymes, permanently deactivating them. Without those enzymes, particularly PTGS2, chemical messengers called prostaglandins can not be produced, and the prostaglandins mediate the inflammatory reaction.
In recent years new drugs designed to affect only PTGS2 have been developed, with the promise of less GI irritation (it turns out that PTGS1 is protective of the GI mucosa) than aspirin which inhibits both. However, it turns out that PTGS1 also produces thromboxane A2 in platelets, causing them to aggregate and thus clot. Blocking PTGS1 reduces the tendency for clotting, and this has cardioprotective effects. It is thought that the vasculature of the body expresses PTGS2, and blocking it only and not PTGS1 as well increases the chance of a coronary clot, or heat attack. All of the so called COX 2 inhibitors except for one have been taken off the market because of this. One is left and is of good benefit for arthritis patients that can not tolerate aspirin.
Aspirin also increases the concentration of nitric oxide (NO) in the body. (Anyone remember from our discussion about nitrogen what, amongst other things, that NO does? Can you say Viagra?) There is some evidence that increased NO levels affect the way the white blood cells work, thus increasing the immune response. There is no clinical evidence that aspirin actually increases the immune response, but this potential sort of turns on its head the old saw that taking aspirin will make a cold last longer.
A very interesting article from last year at Oxford in the UK indicates that daily aspirin has a significant cancer reduction potential. This study, on a population of 25,000 people, indicates that a 10% reduction in prostate cancer rates, a 30% reduction in lung cancer rates, a 40% reduction in bowel cancer rates, and a whopping 60% reduction in esophageal cancer rates. This is a significant study, and those results are exciting. Here is a link to a report on that study.
Some people are sensitive to aspirin, asthmatics being prone to have their condition worsened by taking it. On the other hand, there is evidence that salicylates may be essential human nutrients. Do not worry, there are enough salicylates in fresh fruits and vegetables for you to get your minimum requirement.
Some people are unable to metabolize aspirin quickly enough and can get a toxic reaction from buildup in the system. The classic symptom of aspirin overdose is tinnitus, or ringing in the ears. If you are taking large doses of aspirin and your ears begin to ring, back off some.
The most common adverse effect of aspirin intake is GI bleeding because of the thromboxane inhibition. There are several methods to reduce this, such as buffered aspirin, taking aspirin with Vitamin C, enteric coatings (that do not release the aspirin until it clears the stomach, and by the way does not seem to work), licorice extract, and the amino acid S-adenosyl-methionine. This last approach has been shown to reduce GI bleeding by up to 90%.
The greatest risk posed by aspirin is that of Reye Syndrome in children adolescents. Reye syndrome is sort of a mystery, and the link to aspirin is really not that strong. However, due to the high mortality and morbidity rate of this syndrome, caution is the best action. It is wise to use an ibuprofen preparation for anyone under 19 unless aspirin is specifically indicated (the only indication that I know in that age group is the rather rare inflammatory disease termed Kawasaki Disease). I do not recommend acetaminophen for any reason for any one.
Well, that about does it for this evening. Sorry to be a little late, but I had a busy afternoon. Aspirin is indeed a very useful material and just because it was marketed starting 112 years ago does not make it obsolete.
Well, you have done it again! You have wasted many more einsteins of perfectly good photons reading this sour subject. And even though Michelle Bachman stops her Botox injections when she reads me say it, I always learn much more than I could ever possibly teach by writing this series, so keep those comments, questions, corrections, and other feedback coming. The comments section is usually the best part of my pieces. I shall hand around as long as comments warrant, and shall return tomorrow night after Keith’s (who still has not contacted by about that science adviser spot on Countdown) show.
Warmest regards,
Doc, aka Dr. David W. Smith

4 comments
Skip to comment form
Author
a good, old drug?
Warmest regards,
Doc
who has had a heart attack about five or six years ago (caused by smoking 2 packs a day stiffening his arteries) is big on acetaminophen when he has a cold or other aches and pains.
He’s pretty paranoid about taking ANY over the counter cold medicines fearing they will interact with his other meds. It drives me nuts watching him be miserable every time he gets a cold. He just sort of grinds through them never taking anything (with the exception of tylenol) that helps the rest of us suffer less symptoms.
Are you going to do a companion piece that expands on
?