First, please allow me to apologize for not posting Popular Culture Friday last. I was occupied until late in the day and did not have enough time to write a quality piece for the series, and I would rather post nothing rather than a poor piece. It shall return this coming Friday.
Actually, this is not about soap, but rather synthetic detergents, although we often call then “soap”. In the companion piece to this one from last week, here, the terms are explained in detail.
There are a couple of reasons for using synthetic detergents over actual soap. Part of it is economics, because both vegetable and animal fats, essential ingredients for soap, tend to be fairly high in cost. Most detergents are based on petroleum, so when oil prices are low then can be cheaper to produce than soap. When petroleum is high in cost, then detergents become less economically favorable.
But there are more reasons than economics. Soaps tend to produce lots of foam, and this is not acceptable in many applications. For example, in an automatic dishwasher, the use of soap or a detergent with a high potential for foaming can cause the dishwasher to “foam out”, or fill with foam, reducing the efficiency of the wash and at times escaping the gasket and thus into the floor. The same can be said about the front loading clothes washing machines.
Detergents can be formulated to produce relatively little foam, so can be used in these devices without risk of foaming out. But that is only part of the problem. Last week we went into great detail about how soap interacts with hard water, and this is one of the largest reasons that detergents are often preferred to soap.
Many detergents are much more resistant to the effects of hard water than soaps. This gives them an advantage over soap in hard water areas, since much more extra soap is needed to produce a cleansing effect in hard water than are many detergents. However, detergents are not completely immune to the effects of hard water, so most formulations contain some sort of water softening agent, usually sodium carbonate, to maximize their effects.
The term synthetic detergent is the technical name for these materials, but for simplicity we shall just use the word “detergent”. I have a textbook somewhere around and the author of it insisted using the term “syndet” to signify a synthetic detergent. I found the term to be silly, and have never seen it used anywhere else.
Detergents work in much the same way as soaps do, having a lipophilic and an hydrophilic end for the most part. But while soaps are alkali metal salts of long chain fatty acids, detergents are much more varied as far as their chemistries go. The most common type of detergent used in consumer products are anionic detergents (soaps are also anionic). In an anionic detergent, the detergent part of the molecule has a negative charge (negative ions are called anions) and the charge is balanced with, usually, an alkali metal cation (positive ions are called cations).
The first generation of anionic detergents have pretty much been phased out because they are difficult to break down in sewerage treatment systems. This was a big problem in the late 1960s, when you could see news reports of rivers being covered with thick (I mean many feet think, not like the videos on You Tube) layers of foam. Most anionic detergents are alkylbenzenesulfonates, which sounds like a mouthful but is really not that difficult. Here is a structural diagram of the most commonly used one, sodium dodecylbenzenesulfonate:
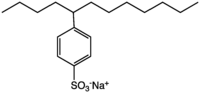
If you look at last week’s piece you can see that this is similar to soap, with a long lipophilic part and then a small, highly charged part. This particular material is used in many laundry products, but not so much in personal car products. Now, when the material is produced commercially there is a mixture of compounds, but this type of detergent behaves on average as if all of the molecules had a 12 carbon chain (hence dodecyl).
The older alkylbenzenesulfonates had branched chains and were difficult to treat as I mentioned before. They are no longer used in the more highly developed nations, but can still be found in developing countries. Here is a representative formula for such a detergent:

This is still a sodium dodecylbenzenesulfonate, (count your carbons), but is not linear like the one above. For personal care products, two other anionic detergents are very commonly used. The first is sodium lauryl sulfate with the following structure:
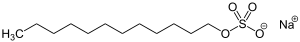
The second one is sodium laureth sulfate, with this structure:

Once again, you have a long lipophilic chain and a highly charged end in both of these. These have no benzene ring, but make excellent suds and so are preferred for things like shampoo where foaming action helps to lift oil and dirt from the scalp and hair. My bottle of Herbal Essences shampoo contains both.
A second type of synthetic detergents are the nonionic detergents. In these, there is no sodium ion to leave the detergent when it is dissolved in water, and no chance of forming a less soluble calcium or magnesium derivative (remember, I told you that most detergents are LESS affected by hard water than soap, but the anionic ones are affected somewhat). A typical example of this class of detergents is Triton X-100 (a tradename of Dow), relatives of which are often used in liquid laundry detergents, along with anionic ones. Here is a structural formula:
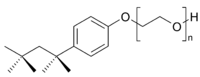
Instead of a highly charged end, this material has one end as a very oxygen rich chain (remember, oxygen hydrogen bonds) and the left end is lipophilic. It the same old story, one end that dissolves in water and the other end that dissolves in fats and oils.
A commonly eaten form of nonionic detergents are the Tweens, aka polysorbates. One common one is Polysorbate 80, and when I looked at the label on my jar of Claussen Whole Kosher Dill pickles, sure enough it is in there. They are used in foods and pharmaceuticals to emulsify ingredients, but are bit too expensive for laundry use. Here is the structure of Polysorbate 80:

The third type of detergent is the cationic detergent. In these, the detergent molecule is positively charged when dissolved in water rather than negatively charged or neutral. The most common one of these is the active ingredient in the modern formulation of Lysol, benzalkonium chloride. (older formulations of Lysol contained cresols, which are pretty toxic and caustic. However, I think that the older formulations worked better, but that is just me). Here is the structural formula for benzalkonium chloride:
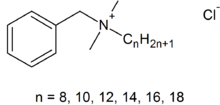
As you might imagine, this material is not as effective a detergent than the ones that we have looked at before, because the charged part of the molecule is near the middle. Its main use is to kill germs on smooth surfaces and on hands (it is an ingredient of several of the more effective hand sanitizers). There are lots of other cationic detergents, but are not used much for actual cleaning in household use.
Interestingly, one of the major household uses of cationic detergents is not for cleaning for for fabric softening. The main cationic used for that is dipalmitoylethyl hydroxyethylmonium methosulfate. I apologize that I could not locate a structural formula for it. It softens fabrics because it caps hydrogen bonding sites on fabric so that the fibres do not tend to attract one another, making the fabric less likely to wrinkle and less harsh feeling.
You can see that from a cleaning product standpoint, the anionic detergents are by far the most widely used, followed by a few of the nonionics. Cationic ones are used for some of their detergent properties, but not for much household cleaning. Now, both the nonionics and cationics have niche uses and lots of highly technical ones, but are not produced in anywhere like the amounts of the anionics. Anionics are produced worldwide on a scale of something like 13 billion pounds annually!
Some products called detergents actually contain no soap or detergent at all. For example, my store brand box of dishwasher detergent states the following ingredients: Sodium carbonate, sodium silicate, and enzymes. The sodium carbonate and to a lesser extent, the sodium silicate, act to saponify the fats in the residue in dirty dishes to soap as discussed last week. The enzymes tend to break down the proteins in the residue to more easily removed materials. By the way, the rinse aid that I add to the reservoir now and then contains a nonionic detergent, so I am really rinsing my dishes in a detergent solution, so I must be eating it the next time that I use then.
Well, you have done it again! You have wasted many einsteins of otherwise perfectly good photons reading this slippery piece! And even though Mittens realizes that he really can not pass as a Tea Party favorite when he reads me say it, I always learn much more than I could possibly hope to teach by writing this series. So, please keep those comments, questions, corrections, and other feedback coming! Tips and recs are also highly treasured.
I shall hang around tonight as long as comments warrant, and shall return tomorrow after Keith’s show for Review Time. Next week we shall tackle another topic, and I have had a request to do a piece on zebras. What do you think? The Countdown folks still need to contact me about that science adviser gig, so please encourage them to do so.
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at
Docudharma, and

3 comments
Author
a slippery subject?
Warmest regards,
Doc
is Ivory. Supposedly 99.44 percent pure soap.
Having grown up using it, no other soap feels like it actually works. My skin may be dry, but at least I feel cleaner anyway. Prob all in my head.
Thanks for another educational post!