Happy New Year to everyone! I hope that 2011 will find you well and prosperous. Last week we began to examine the so called Rare Earth Elements, which turn out to have lots of uses, many of them to do, oddly, with optics in diverse ways. This week we shall finish up the series about them.
I reluctantly admit that I did not take as much time as necessary for the piece last week, mostly because even I did not understand just how important these elements are, both from a modern technological standpoint and also from an historical one for chemistry becoming a modern, “hard”, science. First we shall look into some history, then finish up the elements themselves.
I was amiss for not explaining the origin of the term Rare Earth elements. At the time that they were just beginning to be discovered, an “earth” was synonymous with an “ore”. Thus, the Rare Earths were metal containing ores of unfamiliar elements then. We are talking a timeframe of the early to mid 1800s. It turns out that one of the first sources of these “earths” were found in Sweden, around a town called Ytterby. Note that many of the names of the elements are derivatives of this name.
Actually, these elements are not particularly rare, although at the time of them being named, only a very few sources of ore were known, hence the name. For example, cerium (the most abundant rare earth) is about as common as copper, so many of them are not really rare at all. Now, some of them are comparatively rare in the ores, so several are not as common as others.
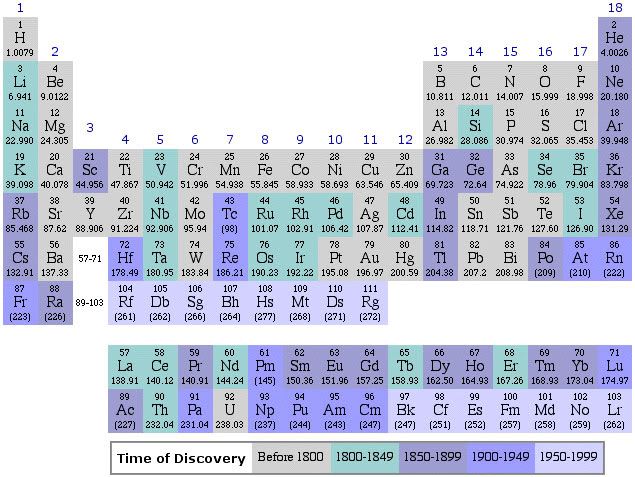
In my piece last week I was also amiss by not inserting a periodic table to help you visualize the electronic structures of these elements. Here it is. Notice that these elements occupy a single space on the main table, with a row near the bottom of it in group 3. Note that some authorities include scandium, Z = 23, and yttrium, Z = 39 with the rare earths. This is because their chemistries are similar to them, but because of atomic radius differences not as close together as what I consider to be the true rare earths.
The distribution of the various elements in the ores is quite interesting. As I mentioned last week, their chemistries are extremely similar, so normal weathering processes do not separate them as efficiently as they separate, say, zinc and lead. Thus, almost all rare earth ores contain all of the elements, but all of the different sources of ore have a unique fingerprint of their elemental composition.
Another interesting fact is that the ones with a Z that is even are almost always more common that either one before or after it with an odd Z. This has to do with nuclear quantum mechanics, and it turns out for the heavier elements a general rule can be stated that elements with an even Z are, for the most part, more stable than those with an odd Z. This gets even more interesting in that, on the whole, elements with an even number of neutrons tend to be more stable than those with an odd number. Thus, heavier atoms with both an odd Z and and even mass number (required to be even if both the number of protons and neutrons are odd) tend to be the most unstable of all. There are exceptions, but the trends are very clear. That is why the rare earth elements are almost always found together, but in strikingly different quantities.
Let us look at a bit of history. The rare earth ores are usually said to be discovered by Carl Axel Arrhenius (only a distant relative, if any at all to the great Swedish chemist, Svete Arrhenius, who live two or three generations later) in a mine Ytterby, Sweden. This was an interesting material, and the chemists, such as they were at the time, tried to see what it was. In time, this “earth” reached the renowned chemist, Johann Gadolin, who examined it in some detail. Remember, one of those elements are named for him. All of this was in a narrow calendar. All of this happened from 1787 to 1803.
Well, what else was happening? Something like the American Revolution, as I recall. I firmly believe that Benj. Franklin was one the the driving forces behind these discoveries, since Franklin had the audacity to say “PUBLISH YOUR WORK, and I have a press ready.”
In any event, the samples finally came to my Ph.D. ancestor, Jöns Jacob Berzelius. He did the best that he could with the technology at the time and found that the rare earth ore had at least two components, later to be found to be elements. Now you see why this is so personal for me. I just wished that Norm had done the certificate thing that Kuroda did. I, with your help, will try to trace my Ph.D. all the way back to Berzelius, and send him a copy of what I intend to do on modern parchment.
In any event, as those ores were more and more examined, it turned out that they just were getting more complex. I have had the same experience. Sometimes it is just better to leave things alone, or to suffer things more complex than you really wanted.
Over 30 years later, Carl Gustav Mosander was keeping up with this work. Whilst I have no direct evidence, he may have been a professor in my lineage. Lineagolists, please help here. Now there were six elements, and it confounded every chemist at the time. How could those elements, with very similar properties, occupy the Periodic Table in the same place? This is where we were in 1842, with six elements that could not be placed in the Table. Yikes!
This continued to be the situation for decades, with new elements claimed and refuted and the exact number of the elements was not clear. It was only after early quantum mechanics became developed that the number of lanathide elements was determined to be 15, since there are 14 f orbital electron spaces, which along with zero comes to 15. Element 61 was not found until after the nuclear age, since it has no stable isotope. This was another tribute to the genius of the Periodic Table, because in its essence it is but a reckoning of the electronic structure of the elements, and that electronic structure is the basis for all of chemistry.
Most of the problem in discovering the elements is that their chemistries are so similar that it is really hard to separate them. That is also why they are always found together in nature, since natural processes also very inefficient at separating them. When modern ion exchange techniques, which are incredibly efficient at separating materials with only very subtle chemistry differences, were developed in the 1940s the pure elements became available in quantities significant for exploitation.
Last week we got through terbium, so let us continue with the uses for the rest of the rare earth metals.
Dysprosium, Z = 66, comes next. It is relatively uncommon, even for a rare earth, and has extremely valuable magnetic properties. When used to replace part of the neodymium in rare earth magnets, it makes those magnets harder to demagnetize. This is useful in high performance electric motors that are subjected to rigorous conditions, like electric automobile motors. There will be an acute shortage of this element if hybrids and plug ins become popular, and likely other magnetic materials will have to be found to substitute for it. It is also used as control rods in nuclear reactors since it is an efficient neutron “sponge”. However, there are other good control rod materials, so I suspect that the magnetic properties of dysprosium will be of chief value in the coming years.
Holmium, Z = 67, is next in the series. Like neodymium and dysprosium, it has extremely useful magnetic properties but is too rare to be used in really large applications. It is used in solid state lasers, particularly for fibre optic communications because holmium based lasers are fairly efficient and operate in the infrared, commonly used for signal transmission. It has also been used as control rods, but being even more expensive than dysprosium has mostly been replaced by cheaper materials.
Erbium, Z = 68, is even more useful as a laser material for communications because its lazing wavelength corresponds to the infrared wavelength where commercially produced fibre optic materials are the most transparent, thus reducing losses. In addition, erbium is not as scarce as some of the other rare earths. It also has another infrared line that is extremely strongly absorbed by water, making it ideal for laser surgery. Formerly, most laser surgery was done with carbon dioxide lasers, but the modern YAG (yittrium aluminum garnet) crystal ones are simpler and more reliable. When you see laser surgery on the TeeVee you also he a green or red spot where the surgery is being done. That is produced by another, low power laser and is used just to assist the surgeon to see where the real, high intensity infrared laser is aimed. It also is used as an alloying agent for some specialty steels.
Thulium, Z = 69, is the rarest of the rare earth metals. Its principal use is in YAG lasers, and they laze in the infrared as well. One of its lines is strongly absorbed by water, making it, like erbium, useful for medical lasers for surgery. A very interesting use for thulium is that when exposed to neutrons in a reactor, it emits X-rays (actually, this is technically incorrect) with a useful lifetime of around a year. This provides a source for X-rays in areas where electricity is not available for medical, dental, and industrial uses. A thulium source consists essentially of a lead can with a lid that can be shuttered open and closed, much more compact than electrically powered X-ray sources. The technical incorrectness arises from the fact that X-rays are not produced by nuclear transitions, gamma rays are. X-rays are produced by electronic transitions. However, the “soft” gamma rays emitted by a thulium source are of lower energy than some “hard” X-rays. In terms of wavelength, X-rays and gamma rays overlap, so the terminology used should reference the process producing them rather than the use. I should clarify the remark about rarity. Promethium is rarer because it has no stable isotope and thus must be produced artificially.
Ytterbium, Z = 70, is also used like thulium for “X-ray” imagining purposes after being irradiated with neutrons in a reactor, but its greatest use is in optics. It is utilized in high power near IR lasers for industrial use. It also has the useful property of changing its electrical resistivity as a function of mechanical stress, making it useful for transducers that monitor stress.
The last rare earth element is lutetium, Z = 71. Now, this is sort of an oddball in that its f shell is filled, so one could argue that since electrons can now go only into the next higher d orbital it really is a “regular” element. However, I take issue with this viewpoint and consider it the last rare earth precisely because it filled its f orbital but since that is a nonvalence orbital that is still inner electron filling, just like the other rare earths. Its chemistry certainly agrees with my view, and so does the International Union of Pure and Applied Chemistry, and I certainly do not want to argue with them. There are not a lot of uses for lutetium, mostly because of its high cost and difficult metallurgy. One major use is as the detector in PET scans, since cost is not much of an object for a device that requires an on-site cyclotron to make the imaging agents.
Just a word about PET (positron emission tomography) to let you know how it works. Basically you take an imaging agent that has a radioactive nucleide that emits positrons. The positrons, which are truly antimatter, immediately combine with regular electrons in your body and the two particles annihilate each other, producing a gamma ray. These gamma rays are then detected and images constructed from the electronic signals. It is almost like an X-ray, except the radiation comes from the inside of you, not passed through you from the outside. The utility of PET is that, depending on the chemical in which the positron emitting nucleus is bound, different parts of the body can be preferentially imaged, depending on where the chemical is preferentially concentrated. Most of the nucleides used only have a half life of a minute or two, so you do not stay radioactive very long. That is also why you have to have a cyclotron on site, since the agents decay too rapidly to transport them off site, with very few exceptions.
This concludes our review of the rare earth elements. As I said last week, these metals are becoming more and more important as new uses are found for them, and are already strategic materials. This presents a geopolitical problem, since China produces over 90% of the rare earths (some figures are as high as 97%) used worldwide. It the Chinese decide to limit production or restrict export, the entire developed world could be in trouble. Look at the diverse uses for these metals, everything from cigarette lighters to lasers to phosphors to magnets to PET scanners. For materials that many of you did not realize even existed, they are essential for modern life.
Fortunately, China does not possess over 90% of the world’s supply of rare earths. The figure that I saw was around 35%, and in fact the United States has quite a good supply for critical uses. It is not that most of the rare earths are actually rare (although as I have indicated, some are), but locations where they are found are extremely limited. As I said earlier, “earth” meant ore at the time of the phase being coined, and there are only a very few ore deposits of them worldwide, but many of them are fairly extensive and were not found until long after the group of elements were named.
Well, you have done it again! You have wasted many more einsteins of perfectly good photons (produced by rare earth phosphors if you use a CRT display but not a LCD or LED one) reading this common talk. And even though Mark Steyn stops acting the buffoon when he reads me say it, I always learn much more than I ever possibly hope to teach by writing this series, so keep those comments, questions, corrections, and other feedback coming. Remember, no science or technology related issue is off topic in the comments. I shall hang around tonight as long as comments warrant, and shall visit again tomorrow evening after Keith’s show for Review Time.
Warmest regards,
Doc
Crossposted at Docudharma.com and at Dailykos.com

1 comments
Author
not so rare things that we use every day?
Warmest regards,
Doc