Before we start, here is an important public service message brought to you by Translator. There is a fraudulent email going around asking that gmail users verify their accounts by the end to the month to avoid suspension. This is a fraud! If you get an email from [email protected], do not respond and delete it.
We take something as mundane as soap way too much for granted. It is not an exaggeration to say that soap has saved more lives over its history than modern medicine has over its history. Of course, soap has a much longer history than modern medicine, but soap is still essential as a medical adjunct.
The actual origin of soap is lost in prehistory. I suspect that the first soap like materials were plant saponins, and we shall get to them in just a bit. Before we get into the nuts and bolts of soap (and by extension detergents), it is important to understand just how these materials work. At first it does not seem to make a whole lot of sense, but as we continue I promise one of those “Aha!” moments. Ready to get going? I am!
Perhaps the best way to understand how soap works is to understand what it NEEDS to do to clean things. If the only “dirt” that we had to wash away were things like actual soil and other water dispersable or soluble materials, water itself would do the trick. For example, if you get your hands or a dish “dirty” with something like salt or sugar, just a water rinse is enough to remove it. But it is much more complicated than that.
The problem arises with hydrophobic “dirt”, or hydrophilic materials carried in hydrophobic matrices. The term hydrophobic means materials that are not wetted or do not not dissolve in water. The term hydrophilic means materials that ARE wetted or DO dissolve in water. Here are a couple of examples:
The archetypical hydrophobic material is grease. Call it what you want, grease, fat, oil, butter, or lard. It is still grease. If you remember from our discussion about trans fats last week, fats are triglycerides, and a typical one is stearin:
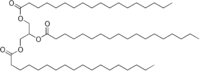
The thing to note is that there is very little oxygen in this molecule, and what there is is NOT bonded to hydrogen. The long hydrocarbon chains are just attracted to water, so grease is insoluble in water. What we need to do is find a was to make it soluble, or at least dispersable, in water so that it can be floated away in water.
The way that soap works is to do just that. Soap (and synthetic detergents) have two different ends on their molecules, one that is hydrophobic (and so is lipophilic, or “fat loving”) and the other hydrophilic. In this way one end of the molecule dissolves in the grease, and the other end dissolves in water. In reality what occurs is that many soap molecules surround a grease particle and their lipophilic “tails” dissolve the grease, and their hydrophilic “heads” dissolve in the water and are then washed away.
Here is a diagram of a micelle:
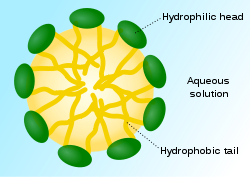
So what do we use to form micelles? This is the counterintuitive part: we use grease as the starting material! Before we get into that, let us looks and the natural saponins which have been used since prehistoric times as detergents. By the way, that term is a very general one for a water active cleaning agent. All soaps are detergents, but not all detergents are soaps.
Here is a structural formula for a typical saponin, solanine:
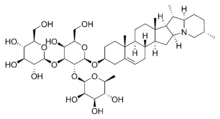
If you look at the left hand side of the molecule, you see three rings that are very rich in -OH (hydroxy) groups. These groups are extremely hydrophilic, and this is why they dissolve in water. As a matter of fact, these rings are sugars, and we all know that ordinary table sugar is very soluble in water. Now, if you look at the right hand side of the molecule you see a complex ring structure that contains no oxygen and certainly no hydroxy groups. This end of the molecule is highly hydrophobic, and will dissolve grease.
A yucca native to the southwestern United States, Yucca elata, goes by the common name of Soaptree Yucca and has roots extremely high in saponins (not solanine). It was used by indigenous peoples to the area as soap. They just beat the root to a pulp in water and the resulting foamy solution was used just like soap water is today. It is a detergent, but not a soap.
The term “soap” is actually quite specific. It can be defined as a metal salt of a fatty acid. For detergent purposes, only soaps where the metal is one of the alkali metals are useful, because most other soaps are not water soluble. For economic reasons, only sodium and to a lesser extent potassium are useful for these soaps because of their low cost relative to other alkali metals. Since sodium is very much cheaper than potassium, almost all soap is sodium soap.
So how do we go from grease to soap? It is really easy. Just as in the old days, almost all soap is made by taking some kind of triglyceride and reacting it with sodium hydroxide. The sodium hydroxide cleaves the bonds betwixt the fatty acids and the glycerol, and water is added during this process so soap is made in an aqueous solution. The old name for sodium hydroxide is lye, and that is why the old timers’ homemade soap was called lye soap. Actually, the modern soapmaking factory uses essentially the same process, although much of the glycerol is removed in factory soap since it is a valuable byproduct, but it was left in homemade soap. The process of converting fats to soaps is called, aptly enough, saponification.
Sodium hydroxide used to be commonly available, but few outlets sell it any more because it is a key ingredient in the process of making methamphetamine. There is actually an extremely simple and cheap method of making sodium hydroxide at home using two common materials, one of which you almost certainly have in your pantry, and the other is used for making pickles. However, I am hesitant to reveal the process because I do not want to assist the methamphetamine makers. I am pretty open minded about what people choose to put in their bodies, but methamphetamine is a scourge.
The real old timers used wood ashes to make soap in place of sodium hydroxide. It turns out that when wood is burnt, the ashes are very high in potassium carbonate, which is similar to lye but not quite as caustic. They would take a wooden barrel and fill it about two thirds of the way with ashes, then extract the ashes with boiling water and stirring. After the ashes settled, they would dip out the potassium carbonate laden water and boil it down to get a concentrated solution. That is actually the source of the name of the element potassium, from “a pot full of ashes”.
When sodium hydroxide is reacted with beef fat, largely stearin, the resulting soap is sodium stearate:
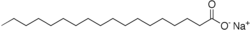
Note that the entire left side of the molecule is only carbon and hydrogen, and thus is extremely lipophilic. The right hand is an extremely hydrophilic group, the carboxyl group associated with a sodium ion. Thus, soap makes excellent micelles and is an extremely efficient detergent with one exception.
That exception is when it is used in areas with hard water. Hard water is water that is high in dissolved calcium, magnesium, and/or iron salts. Why is that? Let us take the example of calcium hardness. When sodium stearate is dissolved in calcium rich water, a calcium ion replaces two sodium ions in two soap molecules, rendering the material insoluble. Here is a structural formula for calcium stearate:
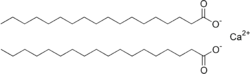
Now instead of having a hydrophilic end and a lipophilic end, both ends are lipophilic. Not only does that make it insoluble in water, it also makes it unable to form micelles, rendering it devoid of detergency. It is possible to add so much soap that all of the calcium is taken out of solution, and then the soap will work. Depending on how hard the water is, this can be quite wasteful of soap.
There are a couple of ways to get around this. One way is to load up the water with extra sodium carbonate (washing soda). Not only is sodium carbonate a fairly effective cleaning agent (is also saponifies fats, but is the “little cousin” of sodium hydroxide in that sense), it also reacts with some of the calcium in the water to precipitate out calcium carbonate, taking calcium ions out of the water.
Another way is to add sodium borate (borax) to the washwater. It works in much the same way as sodium carbonate. There is a product that combines sodium borate and an amino acid to bind calcium in solution rather than precipitate it, but I have never seen it in the store.
A really efficient way to treat hard water is to add phosphate ion, and in particular the tripolyphosphate ion to it. Trisodium phosphate is strongly alkaline and is a better cleaning agent than sodium carbonate and also precipitates out calcium phosphate, thus softening the water. Sodium tripolyphosphate is extremely effective, because it actually forms a cage around calcium ions, keeping them in solution but rendering them unavailable to react with soap. This process is called chelation.
The phosphates have a downside, however, and that is they are nutrients for algae in water. This can cause uncontrolled algal blooms that grow fast then die as the exceed carrying capacity. The resulting decomposition of the dead algae robs water of oxygen, causing fish kills. The excess algal cells also collect at the bottoms of lakes, causing them to become full of silt. Eventually this can cause lakes to become very shallow and also foul. This process is called eutrophication. Thus, many states have banned phosphates in cleaning preparations. These statewide bans have caused phosphates to be effectively eliminated nationwide because it is cheaper to have only one rather than two or three different formulations for the same label. Trisodium phosphate can still be bought at home improvement stores in the paint section and can be added to materials that you can buy.
One important thing about using these materials is to add them to the water BEFORE you add the soap. Once the soap has reacted with calcium, you can not get it back, so treat the water first or you are just wasting your money for the products.
Of course, you can buy a water softener that exchanges calcium ions in water with sodium ones. These are the ones that require that you add salt to the unit periodically since that is the source of sodium ions. These work well. The electric units advertised on The Fox “News” Channel do not remove any calcium or other metals at all, and I debunked them years ago in this series. If I have time I shall try to find the link. Here is the link.
One last thing about hard water. The soap scum that accumulates in showers and bathtubs is primarily calcium soap, and it is hard to get off of surfaces without hard scrubbing, particularly if it has accumulated. I finally hit on the following scheme: I took hydrochloric acid to strip out the surface calcium, reverting the hard material to a free fatty acid. That could be removed with soap, until the next layer of calcium soap was exposed. Then more acid, then more soap. Sometimes this has to be repeated several times, but it does not take really hard scrubbing.
Note that I have not mentioned anything about synthetic detergents in this piece, and that is because it would make it way too long for a single essay. Next week we shall discuss them. I will say that they were developed to overcome some of the drawbacks that actual soap has, particularly the hard water difficulties.
So where do the materials for soap originate? Sodium hydroxide is manufactured from salt and water by an electrolytic process that also produces chlorine. It may well be that the sodium hydroxide was manufactured in the same cell that the chlorine in your bleach was! This makes sodium hydroxide an extremely cheap material since salt and water are cheap, and both the sodium hydroxide and the chlorine are marketable. The grease is another story.
Any triglyceride can be used to make soap, but triglycerides are also important foodstuffs in many cases. Normally, the cheapest triglycerides obtainable are used. A notable exception is Castile soap, which is made from olive oil. However, it is expensive and not seen much these days. Only the most inferior olive oils are generally used for soap. In the old days homemade soap was often made from lard, because pigs are very efficient at converting food to fat, and lard was common. It makes an excellent soap, but is not allowable for practicing Jews and Muslims. The most common animal fat used commercially used for soap is beef tallow. Other commonly used fats are palm oil and palm kernel oil. Both of these plant fats, like beef tallow, are highly saturated and thus not as much in demand for food production as more highly unsaturated ones like soy, canola, and several others.
I am looking at a bar of Irish Spring, and it lists sodium tallowate (beef), sodium palmate (palm), and sodium palmkernalate (palm kernel). These are hardly highly scientific names, but you get the idea. Depending on the location, different fats are used for economic reasons. Each fat has a different sodium hydroxide requirement because, depending on the average molecular weight of the specific fat, more of less hydroxide is required to saponify each molecule of the fat.
One thing that is extremely undesirable is to use too much sodium hydroxide. Doing this not only wastes material, it leads to a soap that contains free sodium hydroxide, making is very irritating to the skin and eyes. Commercial soap is carefully monitored for hydroxide content, and home soap makers can consult any of several web sites that have data indicating how much hydroxide to use per unit mass of fat of a particular kind. If you make your own soap, I suggest that you look up the right amount to use. Just because Great Grandma used a specific recipe does not make it a good recipe.
On the other hand, if not quite enough sodium hydroxide is used, a soap with a slightly reduced cleaning power is the result (because the residual fat acts like soil to the soap that is there), but a much less irritating product is the result. As a matter of fact, my Irish Spring contains hydrogenated tallow acid as a skin conditioner. If you are making your own, it is better to err on the side of too little sodium hydroxide.
So, how was soap invented or discovered? No one know the answer since it is lost in prehistorical times. However, I can offer a speculation, and I think it is a pretty good one. Imagine sitting around the glowing coals roasting a fatty piece of meat in Neolithic times. Some of the fat dripping from the meat finds its way to ashes rather than being burnt up on the coals. Everyone eats and turns in for the night. The next morning it begins to rain and one of the Neolithics notices that the ashes are foaming. People were just as curious then as now, so our distant ancestor starts fooling around with the foaming ashes and finds that the foam and the rain cleaned her or his hands! Eureka! Now how long it took to go from such an accidental discovery to making soap intentionally is anyone’s guess, but I suspect that such an event is quite plausible.
Well, you have done it again! You have wasted many more einsteins of perfectly good photons reading this slippery piece. And even though Darryl Issa realizes that he is playing politics with his investigations when he reads me say it, I always learn much more than I could possible hope to teach writing this series, so keep those comments, questions, corrections, and other feedback coming!
I shall stay around tonight as long as comments warrant, and shall return tomorrow after Keith’s show for Review Time. By the way, he still needs to call me for that Science Adviser gig on countdown. Bill Nye indeed.
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at Daily Kos,
Docucharma, and

1 comments
Author
for keeping it clean?
Warmest regards,
Doc