Oxygen is one of the most fascinating elements for many reasons. Before we get to it, I first want to point out that the column of the periodic table that starts with nitrogen are called pnictogens, whislt the column starting with oxygen are called chalcogens. The term pnictogen is recent, dating form the 1950s. It comes from the Greek plural noun pnikta which means something on the order of “those that are suffocated” in reference to the fact that nitrogen will not support life. The “gen” part is from the Greek gonos, “born” or “generated”.
Chalcogen comes from the ancient Greek chalkos, meaning “ore” and gonos, and in fact an extremely large number of metal ores contain oxygen or sulfur of both. Selenium and tellurium are chalcogens that are often found in gold and silver ores.
Time before last we discussed nitrogen and molecular orbital diagrams for it. If you are not hip to MO diagrams, I suggest you read that part of the link before you try to tackle the MO diagrams for oxygen.
Of all of the substances that we encounter every day, oxygen is the one that is most essential to life. You can go without water for days, without food for weeks, but except in extreme circumstances (most often seen in child drowning and are revived and OK after half an hour or more of immersion) eight minutes without oxygen and you are dead. This is pretty remarkable dependence of a substance in which we should not be able to survive an encounter.
Oxygen gets it name from oxys, meaning “sharp” and gonos again. I love words! I just realized that gonos is also the root for “gonad”, which dovetails nicely with the “born” definition. Oxygen should never had been given that name, because is is based on a false premise. But we must not be too harsh.
The brilliant French chemist Antoine Laurent Lavoisier determined, incorrectly, that oxygen is a constituent of all acids (oxys, “sharp” describes the sour taste of acids). It turns out that not all acids contain oxygen. However, there is more too it.
In aqueous solution, water plays a critical role in stabilizing protons that have to be released for an acid to do acidy things. Without the negative charge of oxygen in water, the very hard (a single charge placed on a very small body, and protons are about as small as they get) positive charge would make the energetics of proton transfer impossible. But since water stablizes those protons by dispersing the charge onto a larger volume, making it “softer”, in a sense all acids DO contain oxygen, at least in aqueous solution.
It is the third most abundant cosmically, and the most common element on our planet. It has one very stable isotope that is “easy” for stars to make, 16O. This is three helium nuclei and helium nuclei are awfully common in stars. The other two stable isotopes are 17O and 18O at about 4 parts per million and 0.2% respectively. The first one is made mostly by the CNO cycle in stars more massive than the sun. The second is formed by alpha capture by a 14N nucleus.
Oxygen is extremely reactive chemically and only the photosynthesis in green plants keeps at at almost 21% or our atmosphere. This is the highest concentration of free oxygen observed in the solar system. In addition to free oxygen, there are quadrillions of tonnes tied up in water, most minerals, biomass, and other reservoirs.
Oxygen has a Pauling electronegativity of 3.44, second only to fluorine. But chlorine has an electronegativity of only 3.16, quite a bit lower than oxygen and everyone knows that to spend even a minute in an atmosphere of 21% chlorine would be fatal. What gives?
It has to do with quantum physics (what does not?). Remember the (faulty) molecular orbital diagram that I sketched for nitrogen last time? Here is one (correct) for oxygen. Note now that all of the 2 sigma and 2 pi orbitals are occupied, like for nitrogen. But we still have two electrons left over, and they must go into one of the pi* antibonding orbitals. Quantum mechanics rules that the two electrons have to go into different orbitals, and that their spin has to be the same (represented by the two uppointing arrows in the pi* MO.
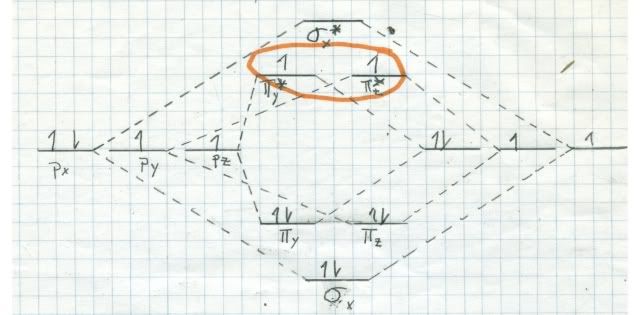
The ramifications of this are enormous. Remember last time we talked about singlets and doublets? Remember, a singlet has all of its electrons paired. A doublet has one unpaired electron. Well, the most stable state of oxygen (called the ground state) is a triplet! That means that there are two unpaired electrons in the same spin quantum state. But it gets better!
It turns out that in the quantum universe, there is a rule about triplets reacting with things. Oxygen is thermodynamically very reactive. That means that the energy release when oxygen reacts with something is large, and nature favors large energy releases. But oxygen is a triplet in the ground state, so it is extremely kinetically unreactive with singlets (one of the rules). That means although the end point is very favorable for oxygen to react, it takes so much energy to get it started that the process is very slow. Reactions betwixt singlets and triplets are called spin forbidden and are very improbable. Look at the reaction coordinate diagram:
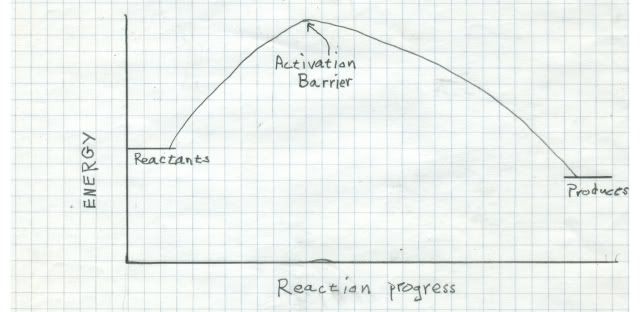
On the left are the energies to two potential reactants. Just to the right is the activation barrier that must be overcome to get the reaction started. If the activation barrier is large relative to the energy release by the reaction, the reaction rate will be very low. That is why we do not burst into flame spontaneously!
If oxygen were a singlet in its ground state, it would burn us like bare fire! We would begin to smoke, but long before we burnt to death we would have died from our lungs being attracted by singlet oxygen. By the way, there are two singlets (all electrons paired) very near to each other in energy, but much higher in energy than triplet oxygen. Here are the MO diagrams for them:
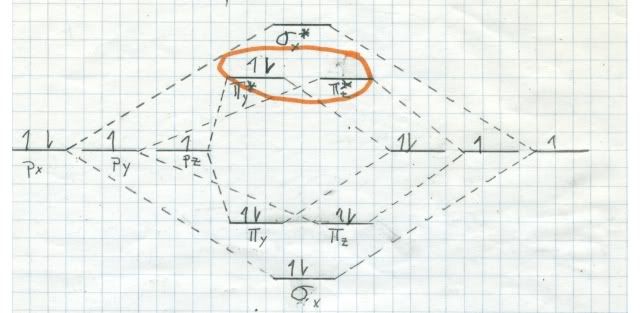
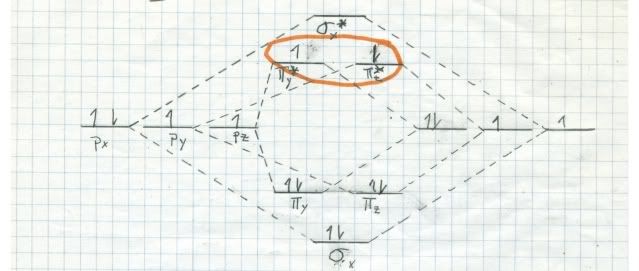
The one at top is a little more stable, since it has both electrons, spins antiparallel, in the same orbital. The other one has the electrons in DIFFERENT orbitals, but with antiparallel spins. It decays into the top one almost instantly. This stuff is bad!
It is not possible to use photons to produce singlet oxygen, because spin forbidden transitions are at the limit of probability. It is possible to produce it chemically, and I had hoped to give you a home chemistry experiment to try, but alas! At the concentrations that chlorine bleach and home hydrogen peroxide come, it is not possible to “see” singlet oxygen. Remember, singlet oxygen is higher in energy than triplet oxygen, and decays to it (slowly) in most cases by releasing a photon in the near ultraviolet. That is a singlet-triplet transition and is highly forbidden. However, when two singlet oxygen molecules approach each other, their highly allowed singlet-singlet interaction allows them to relax and emit a photon of twice the frequency, this time in the visible at around 635 namometers, sort of orange. Quantum mechanics can be fun!
I did a demonstration for Organic Seminar where I produced high concentration of singlet oxygen, using 30% hydrogen peroxide as the oxygen source and elemental bromine as the oxidizer. You could easily see the flashes of orange light as I dropped the bromine from a Pasteur pipette into the hydrogen peroxide. You can do the same thing by using chlorine bleach, but I tried it tonight and you just can not produce enough singlet oxygen in 3% peroxide to make it visible. If anyone knows a way to do it with readily available items, please let me know. I do know that 6% hydrogen peroxide is available at hair product stores, but it is being watched now because the authorities think that terrorists are stupid enough to use the explosive derived from it. It is so unstable that just carrying a jug of it can be enough to detonate it.
In addition to being able to be in either singlet or triplet spectroscopic states, oxygen also forms several allotropes, the most important of which is ozone. Ozone is a molecule consisting of three oxygen atoms, and it is a singlet, so it reacts FAST. If you were in an atmosphere if 21% ozone for a minute, good-bye! We have a love/hate relationship with that molecule. Here is the resonance structure diagram of ozone, and you can see that all of the electrons are paired.
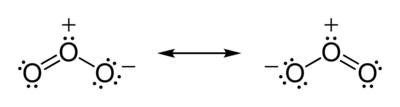
High in the atmosphere, ozone is produced by ultraviolet (UV) irradiation of the little oxygen there. The free radicals formed soon find a triplet oxygen molecule and react with it. Since oxygen molecules are triplets and oxygen atoms are doublets, that reaction is not spin forbidden and happens fast. We really need ozone there, because it absorbs even more UV than oxygen and protects us from excessive levels of UV irradiation.
Its name is more understandable. It comes from the Greek ozein (stinky) and it certainly is, in sort of a refreshing way. It is the smell that you get after a thunderstorm (although some is from nitrogen oxides as well). But better it there than with on us on the surface! However, you can also sometimes smell it around high speed photocopiers where the intense light photolyzes oxygen into oxygen atoms which then react with more oxygen to form ozone.
Ozone is very destructive in the breathing zone. It attacks all forms of organic materials, and is largely responsible for automobile tires to break down and fair. We need LOTS high in the atmosphere, and little in ours. The reason that ozone is so important in the upper atmosphere is that ordinary oxygen absorbs UV only weakly at wavelengths above around 200 nm, whilst ozone absorbs all the way out to 315 nm, and so absorbs much of the most damaging UV that nitrogen and oxygen can not.
Most surface ozone is formed from automobile exhaust, when nitrogen oxides can react with hydrocarbons and atmospheric oxygen to form it, under the influence of sunlight. This is one of the reasons that catalytic converters are required on cars, because they reduce the amount of hydrocarbon emissions. Nitrogen oxide emissions are trickier to eliminate because nitrogen oxides form from direct combination of those elements in the combustion chamber.
Ozone has some important uses even though it is highly reactive. In fact, it is its reactivity that makes it useful. Many of the applications are for killing pathogenic microorganisms. Let us take water treatment as an example.
In most water treatment schemes, after the other purification steps have been taken, chlorine (from cylinders of liquid chlorine) in introduced into the water to kill any microorganisms that might remain. The chlorine, acting as an oxidizing agent, kills the microbes by destroying their envelope. The addition of chlorine can be adjusted to leave a small amount of residual chlorine to keep the water disinfected. However, chlorine has its problems.
For one thing, it reacts with organic materials remaining in the water to form some nasty residuals, trihalomethanes and haloacetic acids. That trihalomethane is chloroform, a known human carcinogen. The higher the concentration of organics in the water, the greater the amount of chlorine required, and the greater the residual concentrations of the bad buggers.
Ozone can be used in place of chlorine, but it has to be made on site for two reasons. The first one is basic chemistry: ozone decays to ordinary oxygen rapidly, and so can not be transported efficiently. The second is that condensed phases of ozone are dangerously and treachourously explosive, so even if it did not decay away, you still could not transport it.
Ozone is bubbled into the water until a specific amount, depending on the chemical analysis of the particular water, has been added. The ozone is normally produced by a silent electrical discharge in ozone generators that are metal tubes with electrodes through which air passes. Some of the oxygen is converted to ozone, and concentrations of up to 6% can be had. Vacuum ultraviolet generators are increasingly being used since they make a product free of nitrogen oxides, unlike the electrical discharge process. However, they are not as efficient, giving only around half a per cent of ozone.
Ozone does not form the bad actors that chlorine does, but can react with bromide (it occurs at rather significant concentrations, depending on the source of some areas) and oxidize it to the carcinogenic bromate ion. Howevery, ALL water has organic materials, and only a relatively small number of water sources contain significant amounts of bromide. The other disadvantage is, because ozone decays to oxygen quickly, residual ozone can not be introduced in the water to maintain disinfection. However, it seems that the advantages of ozone outweigh its disadvantages in comparison with chlorine for this purpose.
I think that this is a convenient place to pause. Next week we shall look at some of the amazingly diverse compounds of oxygen and some of the chemistry behind it.
On a personal note, please join me in wishing The Girl a very happy 20th birthday Tuesday. The cheesecake that I baked for her is cooling in the oven, and I have yet to make the cherry topping (with pie cherries that I picked and canned), but that can wait until tomorrow. The plan is for she and I to celebrate her birthday tomorrow, just us, and then her get to gether with all of the relatives will be on her proper birthday, Tuesday.
Well, you have done it again! You have wasted many more einsteins of perfectly good photons reading this burning piece. And even though Mike Huckabee realizes that his Clint Eastwood/empty chair joke on his Fox “News” Network show was not funny when he reads me say it, I always learn much more that I could ever hope to teach by writing this series, so please keep the comments, corrections, questions, and other feedback coming. Tips and recs are also welcome. I shall remain for Comment Time tonight unless something changes, and plan to return for Review Time Monday night at 9:00 Eastern. Remember, no science or technology question is off topic here.
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at
Docudharma, and

1 comments
Author
supporting combustion?
Warmest regards,
Doc