Element 9, fluorine, is the first of the halogens, from the Greek halos, “salt”, and gonos, “to bring forth”. All of the members of this family tend to form salts with metals, but fluorine is unique amongst the halogens in that it forms compounds with EVERY element ever tried except for helium and neon.
Fluorine is by far the most reactive element, having everything just right for extreme chemical behavior. It is a small atom that forms a small ion. Its electrons are tightly bound in its ionic form, but oddly molecular fluorine has a remarkably weak bond for a halogen, only iodine having a weaker one.
The element has been known in the form of naturally occurring salts since the Middle Ages, when these minerals were used as fluxes in metal smelting. The purpose of a flux is to make the ore and reducing agent mixture easier to melt, thus speeding the reaction since liquid state reactions occur much faster than solid state ones. A secondary use of a flux is to protect the newly won metal from atmospheric oxygen by forming a protective layer that floats on the metal.
The name derives from the neo Latin word fluores, dating from the 1500s. The great British chemist Sir Humphrey Davy came up with the name fluorine, since calcium fluoride, CaF2 was as is a major source of the element. However, the element was not isolated until 1886, due to its extreme reactivity.
Fluorine is not common in space. This is thought to be due to its ease of destruction as it is formed in stars by fusing with either a hydrogen or helium nucleus, a fast process. There are a few explanations that do not have a whole lot of empirical support to explain why there is as much as exists in space. On earth it is rather common, probably because it is so reactive that it formed fairly refractory materials early in the life of our planet.
Except as a flux, fluorine and its compounds were not used very much until the late 19th century, when the Hall-Heroult process for refining aluminum was developed. The only material that they found that would dissolve aluminum oxide was molten cryolite, sodium hexafluoroaluminate, a mineral that occured (the deposit is now worked out) in Greenland. Fortunately, it is possible to make synthetic cryolite so we are not dependent on natural deposits. Huge quantities of inorganic fluorides are used in that industry and in steelmaking.
Fluorine is also used in other industries, in particular the chemical and pharmaceutical industries. Electric power distribution uses quite a bit, and there are many niche uses. Before we get into those details, let us see why fluorine is so reactive and so special. This is going to get sort of Geeky, but that is why we are here.
Here is a really nice video clip about how to built the molecular orbital diagram for molecular fluorine. This is pretty much the same method that I used for the MO diagrams for nitrogen and oxygen. Remember, fluorine has nine electrons altogether, seven of which are valence electrons.
You see that the final result is that fluorine has a single bond betwixt each atom and, since all of the electron are paired, fluorine is a singlet, meaning that it can react with other singlets (and most matter is in the singlet state) without having to enter into a spin forbidden reaction like oxygen has to do (or, actually, oxygen has to absorb extra energy to convert it to the singlet state, which is why things have to be at a high temperature to begin to burn). Not fluorine! Fluorine is so reactive that we would literally burst into flame in an atmosphere of pure fluorine!
Fluorine is such a strong oxidizing agent that WATER burns in fluorine! The overall reaction is probably as follows:
2F2 + 2H20 yields 4HF + O2
You do not need a heat source to do this, merely blowing a gentle jet of fluorine onto water is enough. Since fluorine has an electronegativity of 3.98 and the value for oxygen is “only” 3.44, fluorine has the ability to strip the hydrogen from water and leave oxygen behind. Since we are, to use a term coined by an alien lifeform on Star Trek: The Next Generation, “ugly bags of mostly water”, spontaneous combustion would certainly occur.
So we already know that fluorine is a singlet, is the most electronegative element, and that the fluorine-fluorine bond is comparatively weak. This helps to make the element highly reactive, and there are a couple of other fundamental properties that require quantum theory to describe.
One property that all elements have is something called ionization energy. This is the minimum amount of energy required to remove a single electron from the atom. The ionization energy for fluorine is 1681 kJ/mol, second only to the completely inert gas neon and almost inert gas helium. However, to gain a single electron releases 328 kJ/mol, making it highly favorable for fluorine to abstract electrons from other things. In other words, conversion of a mole of fluorine gas to two moles of fluoride ions releases 656 kJ of energy. This is roughly the same as the kinetic energy of a one ton car traveling at 65 mph. Not bad, since a mole of fluorine gas has a mass of only 38 grams!
Why is this? It has to do with those seven valence electrons. Fluorine needs only one more electron to attain the same electronic configuration as neon, the only known completely inert element. Gaining that single electron gives fluorine an octet of electrons in its L electron shell, the most stable electronic arrangement known. Remember from discussions past that having an octet is almost always the dominant factor in stability situations.
To take that extra electron, fluorine has to acquire a negative charge. It is quite happy to do so, because there is very little electronic shielding from the nuclear charge of 9+. The K shell (or, alternatively, the 1s shell) is filled with two electrons, and this filled shell does shield the 2s and 2p orbitals (making up the L shell) to a small extent. But the other seven electrons are isoenergetic and spatially separate, so those seven negative charges do not shield the nuclear attractive charge at all, so the eighth electron effectively “sees” most of the attraction. This is NOT the case with third row elements. We shall see in the coming weeks that second row elements are almost as different from third row ones as first row ones were from second row ones.
Finally, fluorine is a small atom and a small ion, by far the smallest of any halogen. In fact, only hydrogen has a smaller covalent radius (the average distance from the nucleus to the outermost electron shell in a shared electron environment) at 25 picometers, where that of fluorine is about 60 pm or a little less. The ionic radius of fluorine is 119 pm (ionic radii are often easier to determine precisely than covalent radii), which is still quite small. The ionic radius is larger than the covalent radius because the extra electron repels the other seven, forcing them to expand to accommodate the extra negative charge.
This combination of properties makes the chemical behavior of fluorine unique. If it is so unique, then where do we use it? The truth is, we do not, much, at least the free element. Fluorine is so reactive that only about 1% of fluorite mineral mined is converted to it. Its uses are highly specialized and the plants using it are carefully designed to handle it. Materials compatible with fluorine are few and far between, since it reacts with everything. However, steel can be used, along with aluminum, copper, and a few other metals. This is because these metals instantly react with fluorine to form a metal/fluorine compound that seals the metal surface from further attack.
As a matter of fact, when Moissan first prepared fluorine electolytically, he used platinum/iridium alloy apparatus with stoppers carved from fluorite mineral, calcium fluoride (CaF2). Fluorite rock is fully fluorinated, so further reaction does not happen. Glass is right out, because fluorine instantly attacts it and turns the silicon (the primary ingredient in glass) to silicon tetrafluoride, which is a gas.
About half of the elemental fluorine produced goes to enriching uranium for nuclear power plants, or for more nefarious things. It turns out that uranium forms a compound with fluorine, uranium hexafluoride (UF6) that is a gas above 57 degrees C. In order to separate the useful 235U from the nonfissile 238U this gas is put into centrifuges and spun rapidly. This process takes advantage of another odd quirk of fluorine: in nature there is only one stable isotope, 19F. Thus, the lighter uranium isotope forms uranium hexafluoride with a mass of 349 g/mol where the heavier isotope forms the material with a mass of 352 g/mol, a difference of only 0.0092%. Since the two gases are identical chemically, physical separation is the only way to go.
The centrifuges force the heavier molecules away from the lighter ones, but the difference in mass is so small that thousands of passes have to be made to get adequate separation. Complicating the problem is that the fissile isotope is only about 0.72% natural abundance. For power plants, enrichment up to around 3% to 5% is adequate, but weapons need somewhere around 90% 235U. This is why the enrichment program in Iran is such a big deal, because by far the greatest time and energy requirements have to do with the enrichment step. That is why the US and the former USSR used plutonium in large part for weapons. Plutonium is made from 238U, but since it is a different element, much less energy intense chemical means can be used to separate it from the uranium.
Whilst we are on the subject of isotopes, we already established that fluorine has only one stable isotope, 19F. There are lots of others, but only one useful one, the radioactive 18F. With a half life of about 110 minutes and since it is a positron (antimatter) emitter, it can produce gamma photons when a positron encounters an electron of ordinary matter. This makes it useful in PET scans, where it is incorporated into a molecule much like glucose. Since glucose is ultimately the source of all energy in humans, this material concentrates in tissues with high energy requirements (like tumors).
It turns out that 19F has a nuclear spin of 1/2, is 100% natural abundance, and has a large magnetogyric ratio it a wonderful nucleus for nuclear magnetic resonance analysis. We have talked about this subject before, and sometime soon I shall do another piece on it.
Most of the rest of the elemental fluorine is used to make sulfur hexafluoride, a heavy, rather inert gas. Modern electrical power transmission substations use switching equipment filled with this gas, which has replaced the old liquid filled ones that relied on the toxic and environmentally persistent polychlorinated biphenyls (PCBs). This is because SF6 has a high dielectric constant and so can allow higher voltages to be handled than switches using air.
It is also used for some inert blanketing situations, like magnesium casting. Have you bought those snazzy new double or triple glazed windows to save energy? The chances are high that the spaces betwixt the panes of glass are filled with sulfur hexafluoride. That is because the thermal conductivity of this gas is about 12.1 mW/mK (milliwatts per meter per kelvin), about half that of air at 24 to 25 mW/mK. This slows the passage of heat from one pane to another. On a down note, the 20 year global warming potential for this gas is 8600 times that of carbon dioxide.
By far the most common form of fluorine in industrial use is that of hydrogen fluoride, HF. The water solution of HF is hydrofluoric acid, just like the water solution of HCl is hydrochloric acid. Although HF is still hard to handle, it is a cakewalk compared to elemental fluorine. If kept rigorously dry, it is rather nonreactive because it is not dissociated. Thus, it can be shipped by rail in tanker cars as a liquid under pressure, since its boiling point is around room temperature.
HF has a host of uses, the bulk of it going into fluoronated organic chemicals. The second largest use is to make artificial cryolite, and steel surface treatment, a little to produce water fluoridation chemicals, and even less for catalysts in the petroleum industry.
Ah, those fluoridated organic chemicals! Here is where you will see as much fluorine as you can, but certainly not the free element. There are a host of them, some of which have been found not to be as nice as we once thought. The largest use of these materials is as a refrigerant medium in air conditioning systems. This got us into trouble with the ozone layer.
Back when mechanical refrigeration was first perfected, the working fluids tended to be things like ammonia or sulfur dioxide. They work very well, but are a severe health hazard if the system develops a leak. During the 1920s DuPont was keen to explore fluorine chemistry and developed a series of easily liquified gases that contained only carbon, chlorine, and fluorine. The DuPont trade name for these materials as a group is Freon. They seemed perfect to replace toxic materials since they were cheap, easy to make, inert, and nontoxic. LOTS of chlorofluorocarbons were made. It turns out that this was not a good move.
Chloroflurocarbons are really stable. They are so stable that they get dispersed into the upper atmosphere where the ultraviolet in sunlight breaks them down into atoms. The fluorine atoms are not that a big of a deal, but the chlorine atoms react with ozone irreversibly, converting it to normal oxygen. The insidious thing is that these atoms are regenerated, forming a chain reaction where one chlorine atom can destroy thousands or more ozone molecules. It works like this:
CCl2F2 + UV radiation yields CClF2. + Cl., where the dots mean unpaired electrons.
Next, Cl. + O3 yields ClO. + O2
Then, ClO. + O3 yields 2O2 + Cl.
and the cycle continues until two chlorine atoms combine to break the chain. However, those are rapidly dissociated back to the two atoms by the UV. It is actually a bit more complicated because there are other unpaired electron species in addition to chlorine.
Many CFCs have been banned, and others are being phased out rapidly. Not wanting to go back to ammonia, the chemical companies began producing hydrochlorofluorocarbons, containing less chlorine and/or fluorine and leaving some hydrogen atoms on the molecule. These materials are much less stable than chlorofluorocarbons and the idea was that they would decompose before they got near the ozone. They are not perfect, but better. They still have ozone depletion potential, and now those are being phased out in favor of hydrofluorocarbons, containing no chlorine at all.
Those are much better for the ozone, but they are also fairly stable and potent greenhouse gases! Sometimes you can not win for losing. Ammonia is sounding better and better, especially with technology to sound an audible alarm in case your refrigerator developed an ammonia leak.
The next largest use for fluorine is in plastics and other fluoropolymers. The most widely known one is polytetrafluoroethylene (PTFE), with the DuPont trade name of Teflon. It was discovered quite by accident in 1938 and its unusual properties immediately attracted attention. It is almost completely inert chemically. I shall tell you a funny story about that in a bit. It is also the conventional material with the lowest known coefficient of friction, and I am told that it is the only substance that a gecko can not climb. Now you know what to put to keep insurance people away!
Since it is completely fluorinated, fluorine and reactive fluorine containing compounds will not attack it. Thus, it is essential for making seals and the like for uranium enrichment, sort of fortuitous since we sort of needed to do that starting in 1942 or so.
PTFE is used in many applications, from your cookware to make it nonstick (I am not a big fan of PTFE coated cookware, by the way), to the little tape that you can use to seal pipe threads, to industrial filters. It turns out that it is easy to introduce pretty uniform sized holes (microscopic) in PTFE films, and they make outstandingly efficient and long lived filters. The caveat is that you can not filter water solutions with them because PTFE is one of the most hydrophopic substances known.
However, this property makes it perfect for the rain barrier Gore-Tex. That is just a PTFE film with lots of tiny holes in it. Liquid water will not wet it, so it can not pass, but water vapor molecules are small enough to make it permeable to them, so, unlike in rubberized rain gear, your sweat can pass out of the garment but liquid water can not pass into it.
When I worked for the Army in the pyrotechnics laboratory, we had a material called “Mag-Tef” which was a sheet of PTFE to which a sheet of magnesium was bonded. When ignited, the fluorine in the PTFE burns the magnesium, giving off lots of heat. It makes a good incendiary, and because of the copious amounts of IR emitted are used for decoy missiles in aircraft defense.
In the laboratory one very common use for PTFE is coating magnetic stir bars. Since PTFE is almost completely inert, and does not scratch glass, it is really good for that purpouse, unless you are making Rieke copper. Rieke copper is an extremely finely divided copper powder used as a catalyst. You make it by dissolving copper iodide in dioxane, then reducing the iodide to the metal with metallic sodium. I put the proper ingredients together in a three neck flask, started the stirrer, and applied the heating mantle. Nothing happened and I got hungry and went for lunch.
Upon my return I went to check my process. I had it under a nitrogen blanket through the reflux condenser to keep oxygen from spoiling the active copper, but when I returned it was just gone. The apparatus was there, but the flask was empty. One of the stoppers (the one in the right neck) was gone, too. At first I thought that one of my fellow graduate students was playing a prank, but the unspoken law about playing pranks is NEVER to mess with someone’s experiment. I looked up at the ceiling, and there was my Rieke copper: a copper red stain of reactants and product on the ten foot high ceiling.
Here is what happened. Everything was cool until the temperature of the dioxane, with a boiling point of 101 degrees C, exceeded 98 degrees C, the melting point of sodium. As soon as the sodium melted, the stirrer whipped it into tiny particles, increasing its surface area by probably three or four orders of magnitude. The molten sodium particles were burned by the fluorine in the PTFE coated stir bar, and the entire contents of the flask were expelled out of the path of least resistance, in this case the right neck of the flask. I would bet that the whole think happened in milliseconds.
I was really lucky that all that was lost was the stopper and the contents of the flask. That could have started a catastrophic fire, because dioxane is flammable and potassium spontaneously can become alit in air. The next time I made Rieke copper I used a glass coated stir bar! As the months wore on, the reddish stain took on the beautiful green patina of an old copper roof, a constant reminder to me to do more research before I started doing things.
Another, sort of related set of compounds are the perfluorinated organic compounds. These materials, like PTFE, have all of their available carbon/hydrogen bonds replaced with carbon/fluorine ones. These materials are quite different in properties from their hydrogen homologues and have many useful properties. Some of them are highly resistant to high temperatures, and so are used on gaskets in demanding situations. I have used many a graphite filled Viton seal for capillary column GCMS. Some are not polymers but just fair sized molecules. Here are a couple.
Perfluorooctanesulfonic acid (PFOS), the structure of which is shown below, was used in the product called Scotchgard until fairly recently.
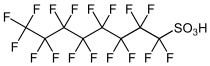
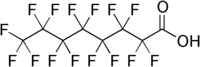
There are a couple of related ones, such as perfluorooctanoic acid (PFOA),
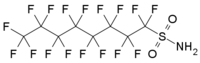
and perfluorooctanesulfonamide (PFSOA).
The problem with these materials is that they bioaccumulate, and the best evidence indicates that enough can be accumulated to have adverse health effects. Scotchgard at one time or another (the formulations have changed over the years) contained one or all of these materials.
The produce has been reformulated with the active ingredient being perfluorobutanesulfonic acid (PFBS).
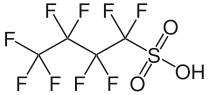
Tests indicate that the human half life of this material is only about a month, where that for PFOS is on the order of five and a half years. I am not so sure that this is a panacea, but it is a step in the right direction.
How do these materials work to make fabric water resistant and stain resistant? It is a pretty neat trick. All long chain or polymeric perfluoro organic compounds are quite hydrophobic, literally meaning “water fearing”, and that is an apt description. You just can not wet the molecules, except on the oxygen rich hydrophilic “head”. This head can form hydrogen bonds with other, like structures, and it turns out that most fabrics contain lots of centers for hydrogen bonding. Let us treat a piece of cotton. Cotton is almost pure cellulose
, a high polymer of the sugar glucose. Here is the structural formula:
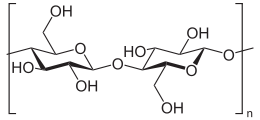
In cotton, n can range from around 400 to 5000, so we are looking at long chains. In real fibres, these chains hydrogen bond with each other to form masses large enough to be seen, as in a single cotton fibre, much wider than a single molecular chain. That looks something like this, where the hydrogen bonds are indicated by the dotted lines:
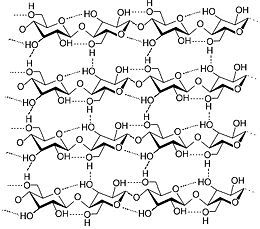
When we treat the fabric with Scotchgard, some of the hydrogen bonds at the surface of a strand are broken and new hydrogen bonds betwixt the, say, PFBS “head” and the fabric are formed, locking the material in place. If applied thickly enough, the long “tails” of the molecules get in each others’ way and they essentially sort of stand up over the surface of the fabric, forming a sort of impervious barrier to water and other hydrophilic materials, and most dirt and staining materials contain water. Thus, it like having a microscopic umbrella over the fabric, and water (and stains for the most part) just stand on the surface, awaiting for you to wipe them away with a more hydrophilic substrate. One note: it does not work to prevent greasy stains, because by definition if something is hydrophobic, it is lipophilic, of a lover of fats.
I could keep going, but just want to point out some of the differences betwixt fluorine and the other halogens. Remember I said earlier that second row elements often have properties quite different than those of the third and following rows. The reason is quantum mechanical for the most part. Second row elements are the lightest of each family (excluding group 1 and group 18, because of hydrogen and helium), so show quantum effects more strongly than heavier ones in many cases. They are also considerably smaller than atoms from later rows. But the greatest difference is that second row elements have only s and p orbitals. Beginning with the third row, d orbitals are possible, and that can make their chemistry more complex. Here is an example.
The solubilities of the chloride, bromide, and iodide of calcium are 74.5, 143, and 66 grams per 100 mL of water, respectively. That is pretty soluble! The solubility of calcium fluoride is 0.0016 g/100mL, almost 47,000 times less than the chloride. That is some difference.
In the title I said that fluorine is something that you have never seen. I mean elemental fluorine, not Teflon. I have been a professional chemist near all of my life, and I have never seen elemental fluorine, and I would wager that no more than one reader has, either, if even one. I have seen elemental americium, but never fluorine. Hell, I did not like handling hydrofluoric acid! That reminds me of one other thing about hydrofluoric acid. Again, F is aberrant compared to other halogens. All of the other haloacids are completely ionized in dilute water solution, but HF is not, and thus is considered a weak acid. That does not mean that it can not hurt you, merely that it is ionized about as much as vinegar. The reason for that is that HF can hydrogen bond with not only water, but itself as well, and this property to accept hydrogen bonds is shared only with nitrogen and oxygen. This bonding tends to make HF form aggregates of rather complex structure and makes it energetically favorable to keep the protons out of the aqueous phase.
Since hydrofluoric acid, like fluorine, turns glass to gas, shipping it in laboratory quantities was sort of difficult until the advent of polyethylene plastic bottles. Before then it was usually supplied in lead bottles (with contamination from lead) or in, egad, paraffin wax bottles for high purity material! Can you imagine how difficult working with a wax bottle must have been? I have lived in places in the south that would challenge the structural integrity of the paraffin wax just with ambient temperatures!
Well, you have done it again! You have wasted many more einsteins of perfectly good photons reading this reactive piece! And even that dolt John Bolton realizes that he used an evil slur on the Fox “News” Channel tonight when he called the administration’s response to the killings of members of our diplomatic corps “limp wristed” when he reads me say it, I always learn much more than I could possibly hope to teach by writing this series. Therefore, please keep those questions, comments, corrections, and other feedback coming. Tips and recs are also always highly appreciated. Remember, no science or technology issue is off topic in the comments.
I shall remain for Comment Time as long a comments warrant, unless I get an invitation to visit someone, and in that case I will let you know. Towards that end, please keep the space after my personalized tip jar clear so such announcements will appear at the top of the comments. I very much appreciate it. I shall return tomorrow evening at around 9:00 Eastern to cover any comments that come in after I retire tonight.
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at
Docudharma, and

1 comments
Author
the most reactive element?
Warmest regards,
Doc