(2 pm. – promoted by ek hornbeck)
 This is a series of diaries focused on the World Health Organization Neglected Tropical Diseases Program. I initially wrote a diary about Dengue Fever that had hospitalized Salon columnist and constitutional lawyer, Glenn Greenwald. The second diary briefly introduced the other diseases on the WHO list.
This is a series of diaries focused on the World Health Organization Neglected Tropical Diseases Program. I initially wrote a diary about Dengue Fever that had hospitalized Salon columnist and constitutional lawyer, Glenn Greenwald. The second diary briefly introduced the other diseases on the WHO list.
This diary will focus in Human African trypanosomiasis HAT, or sleeping sickness, parasitic disease transmitted by the bite of the ‘Glossina’ insect, commonly known as the tsetse fly infected with a protozoa of the species Trypanosoma brucei. The flagellate protozoan Trypanosoma brucei exists in 2 morphologically identical subspecies: Trypanosoma brucei rhodesiense (East African or Rhodesian African trypanosomiasis) and Trypanosoma brucei gambiense (West African or Gambian African trypanosomiasis).
Tsetse flies are found in 36 countries in sub-Saharan Africa, putting 60 million people at risk. The disease affects mostly poor populations living in remote rural areas of Africa. Untreated, it is usually fatal. Travelers also risk becoming infected if they venture through regions where the insect is common. Generally, the disease is not found in urban areas.
Sleeping Sickness is the deadliest disease in the world. Without treatment, the parasites kill.
Symptoms
HAT symptoms occur, generally, within one to three weeks of infection and in two stages. The first stage presents with non-specific symptoms such as fever, headache, joint pains and weakness. This stage is difficult to diagnose but relatively easy to treat. If untreated the parasite invades the infected person’s central nervous system and the second stage sets in.
The term “sleeping sickness” comes from the symptoms of the neurological phase. The symptoms include confusion, reduced coordination, and disruption of the sleep cycle, with bouts of fatigue punctuated with manic periods leading to daytime slumber and night-time insomnia. Without treatment, the disease is invariably fatal, with progressive mental deterioration leading to coma and death. Damage caused in the neurological phase is irreversible.
Diagnosis
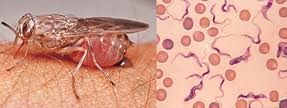 The diagnosis rests on finding the parasite in body fluid or tissue by microscopy. Patient samples that can be used for diagnosis include chancre fluid, lymph node aspirates, blood, bone marrow, and, during the neurological stage, cerebrospinal fluid. It is important to differentiate between the two parasites that cause the disease. T. b. rhodesiense parasites can easily be found in blood while it is often difficult to detect T. b. gambiense in blood. There is a serological test used outside the United States that is used for screening. It’s not always reliable, so the definitive diagnosis rests on seeing the parasite in the blood or tissue sample under a microscope.
The diagnosis rests on finding the parasite in body fluid or tissue by microscopy. Patient samples that can be used for diagnosis include chancre fluid, lymph node aspirates, blood, bone marrow, and, during the neurological stage, cerebrospinal fluid. It is important to differentiate between the two parasites that cause the disease. T. b. rhodesiense parasites can easily be found in blood while it is often difficult to detect T. b. gambiense in blood. There is a serological test used outside the United States that is used for screening. It’s not always reliable, so the definitive diagnosis rests on seeing the parasite in the blood or tissue sample under a microscope.
Treatment
The specific drug and treatment course will depend on which of the two parasites is causing the infection and the disease stage, specifically if it has passed into the central nervous system.
Pentamidine, which is the recommended drug for first stage T. b. gambiense infection, is widely available in the U.S. It is given by intravenous infusion over two hours or by intramuscular injection. Pentamidine is widely available in the U.S. The other drugs (suramin, melarsoprol, eflornithine, and nifurtimox) used to treat HAT are available in the U.S. only from the CDC. Physicians can consult with CDC staff for advice on diagnosis and management and to obtain otherwise unavailable treatment drug.
Suramin is used to treat first stage T. b. rhodesiense. Suramin is also effective against T. b. gambiense, but it is not often used because severe reactions occur.
Most patients respond well to treatment and recover fully when the disease is caught in this stage. The complications come with treating HAT in the second stage.
Second stage T. b. gambiense is treated with eflornithine, which is given in 4 intravenous infusions daily for 14 days. Eflornithine is highly effective, but the difficulty in administering 4 infusions daily in rural African facilities has led to the use of eflornithine (dosed less frequently) in combination with nifurtimox. The efficacy of the combination regimen appears to be at least as high as eflornithine monotherapy. Eflornithine is not effective against T. b. rhodesiense and it is not recommended for treating the East African form of the disease. Melarsoprol, an arsenic based compound, is the only drug available for treating second stage T. b. rhodesiense. Adverse reactions to melarsoprol can be severe and life-threatening. Melarsoprol resistance has become a concern in the Congo and Uganda with up to 30% of cases do not respond to the drug.
A little side story of Eflornithine and the fight that WHO and an NGO waged to get it produced. The drug was originally developed as a cancer treatment by Merrell Dow Research Institute in the late ’70’s. It wasn’t very effective as a cancer treatment but was found to reduce hair growth and, inadvertently, very a effective treatment for HAT. Eventually, it was developed and marketed as a prescription cream, Vaniqa, to treat women with excessive facial hair by the Gillette company.
The drug was registered for the treatment of gambiense HAT in 1990. However, in 1995 Aventis (now Sanofi-Aventis) stopped producing the drug, whose main market was African countries, because it didn’t make a profit. Production for the drug requires a separate facility because the process is very corrosive.
In 2001, Aventis (now Sanofi-Aventis) and the WHO formed a five-year partnership, during which more than 320,000 vials of pentamidine, over 420,000 vials of melarsoprol, and over 200,000 bottles of eflornithine were produced by Sanofi-Aventis, to be given to the WHO and distributed by the association Médecins Sans Frontières in countries where the sleeping sickness is endemic.
According to Médecins Sans Frontières, this only happened after “years of international pressure”, and coinciding with the period when media attention was generated because of the launch of the eflornithine-based product, Vaniqa, geared to prevention of facial-hair in women), while its life-saving formulation was not being produced.
From 2001, when production was restarted, through 2006, 14 million diagnoses were made. This greatly contributed to stemming the spread of sleeping sickness, and to saving nearly 110,000 lives. This changed the epidemiological profile of the disease, meaning that eliminating it altogether can now be envisaged.
There is no vaccine or drug for prophylaxis against African trypanosomiasis. Preventive measures are aimed at minimizing contact with tsetse flies. Local residents are usually aware of the areas that are heavily infested and they can provide advice about places to avoid. Other helpful measures include:
Wear long-sleeved shirts and pants of medium-weight material in neutral colors that blend with the background environment. Tsetse flies are attracted to bright or dark colors, and they can bite through lightweight clothing. Inspect vehicles before entering. The flies are attracted to the motion and dust from moving vehicles. Avoid bushes. The tsetse fly is less active during the hottest part of the day but will bite if disturbed. Use insect repellent. Permethrin-impregnated clothing and insect repellent have not been proved to be particularly effective against tsetse flies, but they will prevent other insect bites that can cause illness. Control of African trypanosomiasis rests on two strategies: reducing the disease reservoir and controlling the tsetse fly vector. Because humans are the significant disease reservoir for T. b. gambiense, the main control strategy for this subspecies is active case-finding through population screening, followed by treatment of the infected persons that are identified. Tsetse fly traps are sometimes used as an adjunct. Reducing the reservoir of infection is more difficult for T. b. rhodesiense, since there are a variety of animal hosts. Vector control is the primary strategy in use. This is usually done with traps or screens, in combination with insecticides and odors that attract the flies.
Sources for this article:
WHO: Human African Trypanosomiasis
Médecins Sans Frontières (MSF) and Sleeping Sickness
MedicineNet; African sleeping sickness

1 comments
Author