Everyone is exposed to elemental nitrogen every day. Since it makes up about 78% of the atmosphere, it is impossible to avoid. It is nontoxic, so being impossible to avoid is not in this case a problem.
Actually, it is good thing that nitrogen comprises that much of the atmosphere. If the atmosphere were much richer in oxygen than it is (around 21%, the rest carbon dioxide and a few others), it would be impossible to fight fires. More on that later.
In addition to being an inert diluent to oxygen in the atmosphere, nitrogen is also essential for life for many reasons, and we shall examine some of them in a bit. It is also an essential building block for many important industrial materials and for fertilizers for plants. Come with us after the fold and we shall examine this important element.
Nitrogen has an atomic number (technically called Z) of 7, making it a second row element. This is important from quantum mechanical aspects, since second row elements can have only s and p orbitals. This is important, and we shall talk about it later. The most common isotope of nitrogen is N-14, with seven protons and seven neutrons in the nucleus. N-15 also exists to the extent of about 0.36% of all natural nitrogen. Radioactive isotopes can be prepared, but all have fairly short half lives and are not used for much.
There are lots of uses for elemental nitrogen. It is used as a cheap inert gas blanket for many chemical processes that are sensitive to oxygen when it can be used in place of the more expensive (but more inert) argon. I used it extensively as a carrier gas for gas chromatography with a flame ionization detector because it is much cheaper than helium, preferred for thermal conductivity detectors and for mass spectrometers. It is extremely cheap and very safe to use, because it is made from nothing but air and because it is not toxic at all (with one notable exception), but does act as a simple asphyxiant.
Liquid nitrogen is used as an easy material for making things very cold. It boils at 77 kelvins (-196 degrees C), so is very cold indeed. I used to use it a lot in the laboratory for many things. It is the medium in which frozen embryos are kept, and also semen for artificial insemination for both man and beast. As a matter of fact, when I was in graduate school we bought our liquid nitrogen from a bull semen supplier. Some avant garde cooks are using it for special effects, and you can make ice cream with it FAST.
You have to be careful with it, though, because it will freeze tissue and cause serious frostbite, but, contrary to popular belief, NOT instantly. As a demonstration, I have many times dipped a finger into it for several seconds at a time, with no ill effect. The reason is that the temperature differential betwixt liquid nitrogen and finger temperature is around 230 degrees, so the finger is incredibly hot compared to the liquid nitrogen, causing it to boil around the finger, so only relatively warm nitrogen gas touches it, for a while. It is sort of akin to putting a red hot piece of metal in water: the liquid really does not touch the metal for a little while. It also has a much lower heat capacity and much lower enthalpy of vaporization than does water, so a four second dip does not any damage at all to a finger.
If left in it long enough to come to 77 K (the degree symbol is not used in that system), matter becomes unusual. If you ever happen on to some liquid nitrogen, take a rubber band, and with tongs, dip in into the cold material. After the boiling stops, then fling the rubber band onto a hard surface. It will shatter like glass! You can also drive a sharp, thin nail into soft wood with a properly treated banana!
Elemental nitrogen is incredibly stable. To dissociate a mole of nitrogen molecules to atoms requires around 942 kilojoules, while to do the same for oxygen requires only around 494 kJ. As a matter of fact, the chemical bond betwixt the two nitrogen atoms is amongst the strongest known.
Why is that? To give a comprehensive answer requires some rather deep quantum mechanical treatment, but in a nutshell it is because atomic nitrogen has seven electrons. Two of those are in the 1s shell, and are not available for chemistry. Two more reside in the 2s shell, and are also not available for chemistry. The remaining three electrons are in the 2p orbitals, and by chance there are three 2p orbitals, px, py, and pz. Unlike s orbitals, which are spherical and thus nondirectional, the p orbitals have direction, as represented by the Cartesian representations x, y, and z. It also turns out that each of these atomic orbitals (AOs) can be added (both physically and mathematically) to form six (sum and difference, in a manner of speaking) molecular orbitals (MOs).
Three of the six MOs are more stable than the constituent AOs and are called bonding orbitals, and the three other ones are LESS stable than the constituent AOs and are called antibonding orbitals. Using the aufbau principle, we add electrons to the most stable orbitals first. Since we have six electrons (each nitrogen has three to contribute), we soon see that all three bonding orbitals are completely filled (a bonding or an antibonding orbital can contain zero, one, or two electrons) with no electrons left. Thus, nitrogen has three bonds betwixt the two atoms. The two px AOs are directed towards each other, and form a sigma bond directly betwixt the two nitrogen atoms. This is the strongest of the three bonds. The other two AOs are directed to the sides of the molecule and form MOs outside of the line connecting the two atoms, called pi bonds. They are not quite so strong as sigma bonds, but are significantly strong. Thus, nitrogen is said to have a triple bond. Chemists immediately recognize that triple bonds consist of one sigma bond and two pi bonds.
Now, let us look at oxygen. For oxygen, Z = 8, so now we have four electrons that are available for chemistry from each atom, for a total of eight. When we build the MOs for oxygen, they look just like the ones for nitrogen, but we have two electrons left over with respect to nitrogen. They have to go somewhere! The only alternative is to place them in the lowest energy pi antibonding MO, where they pretty much cancel the attraction of one of the two bonding pi MOs. Thus, oxygen effectively has only a double bond, since one of the two pi bonding MOs has been neutralized by the new antibonding pi MO. I know that this is Geeky, but it is important. Here is a rough hand drawn representation of the discussion. It is entirely from memory. I did lots of them for my dissertation for more complex systems using a Rapidograph pen with India ink, on glossy paper. Computer graphics were just then beginning to be available, and I could not afford the software. Anyway, at the time, the hand drawn ones (using proper templates) looked better than the ragged lines that were state of the art for Commodore computers then.
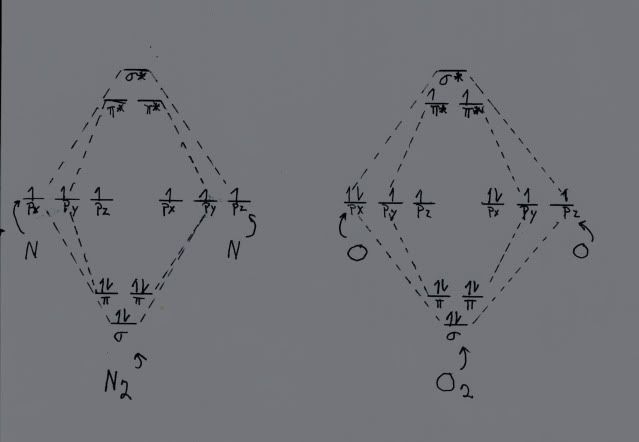
Here are two MO drawings, one for nitrogen and one for oxygen. Note that the left one, nitrogen, shows in the middle the three p orbitals for nitrogen, for two nitrogen atoms, giving a total of six p orbitals. These six AOs sum to six MOs, three lower in energy than the degenerate AOs, and three (denoted by the *) higher. Also note that there is a single electron in each AO. Using the aufbau principle, we start filling the MOs with the lowest energy one. The sigma orbital gets two electrons, denoted by the arrows one up and one down, since the electrons are spin paired. The next two bonding orbtials, the pi ones, also get two electrons each, paired. Thus we have three electrons pairs in bonding orbitals, forming a triple bond, one sigma bond and two pi ones.
The situation is similar for oxygen, except instead of three valence electrons, oxygen has FOUR, denoted by the paired electrons in the px ones. We add electrons just like we did with nitrogen, except when the three bonding orbitals are filled we still have two electrons left. Each antibonding pi orbital gets one electron, thus canceling out one of the three bonding orbitals, so oxygen has a double, rather than a triple, bond. This is also why oxygen is paramagnetic and why we do not burst into flame. If you carry it to fluorine, with FIVE valence electrons, BOTH pi antibonding orbitals are filled, and indeed fluorine has only a single bond. In the case of neon, with SIX valence electrons, the sigma antibonding orbital is also filled, giving a bond order of zero. As a matter of fact, neon and all of the noble gases exist as single atoms because there is no energy advantage to form bonds.
Now with quantum chemistry out of the way, let us look at the history of our knowledge of nitrogen. Until around the time of the American Revolution, little was known about chemistry. Finally, some new thinkers started to experiment and found that air was not an element, but acutally a mixture of several gases. Joseph Priestly, a translocated Englishman to America, is pretty much accepted as discovering oxygen, but he did not know what he had found. What he did know was that the gas given off by, as I recall, spearmint plants grown under a glass made a candle burn extremely brightly and that mice, confined to an environment containing this gas lived much longer than ones confined to a similar environment containing regular air did, until they suffocated. This is actually, although brutal sounding by modern standards, one of the first examples of a controlled experiment, essential for the scientific method.
I wrote a piece about Phlogiston many years ago, but it is still here somewhere. Without going into detail, Priestly believed that bunk theory, and called oxygen Dephlogistonicated Air (all gases were called “airs” at the time). It fit the theory at the time.
The brilliant French chemist, probably the first chemist as known in modern terms, Antoine Lavoisier, did the critical experiments. He showed that the atmosphere was a mixture of two gases, what we now call nitrogen and oxygen. He also assigned them to be in a class of what we now call elements, quite different from the Greek ones of earth, air, fire, and water. Lavoisier was onto something, but was poorly armed with theory. However, he actually pretty much, to use and analogy, was the grader that provided the roadbed for the pavement of modern thought.
Unfortunately, he was an aristocrat in France at a really bad time. I am paraphrasing, but at least one person said that, due to the use of the guillotine on him,
In one second the mind of the century was severed.
That is only one reason that I am not a big capital punishment supporter. There are lots more, but that is off topic here.
Lavoisier called called nitrogen azote, mean “without life”, since it would not support respiration. We now know that nitrogen is essential for life, just not in its elemental form. Nitrogen is an essential constituent of amino acids, which in turn form proteins, without which life as we know it is impossible. That word continues to be used for compounds in which three nitrogen atoms align by cumulated double bonds to form the azide ion, N3–, like hydrogen azide, HN3. Most azides are both toxic and explosive. By the way, if you have an automobile with an air bag, the chances are that it is loaded with sodium azide (NaN3, which explodes readily from an electrical impulse from sensors that detect collisions. The major product is harmless nitrogen gas, and it inflates the bag FAST. It has also been used as rodent poison.
First, we should look at some of its simple, common compounds. In addition to elemental nitrogen in the atmosphere, nitrogen forms a number of binary compounds with oxygen. Both of those elements are interesting, since they are both second row and highly electronegative, but oxygen is more electronegative than is nitrogen. Electronegativity is, in basic terms, the tendency for an atom to attract electrons, either ones in free space or from other atoms. There are a couple of scales for it, but the Pauling one, named in honor of the two time Nobel laureate Linus Pauling is the one that I prefer. On that scale, elements with a number of 2 or greater tend to attract electrons, and ones with a number less than two tend to donate them.
The extremes from from 0.7 (for francium, the least electronegative element known) to 4.0 (for fluorine, the most electronegative element known and also a second row one). Nitrogen is rated at 3.0, and oxygen at 3.5. In general, the further away from around 2, the greater the reactivity of the ATOMS, not necessarily the MOLECULES of elements are. The Noble Gases are arbitrarily assigned a value of zero, but that is for other purposes, to be treated in a separate post. In any event, the larger the difference in electronegativity betwixt two atoms, the more ionic the bond is.
Since nitrogen and oxygen are fairly close in values, their bonds are more covalent than ionic. That means that instead of transferring electrons from one atom to another, they are more of less shared. Thus, in either molecular oxygen (O2) or molecular nitrogen (N2), the difference in electronegativity is zero, so on a time averaged scale, all of the valence electrons are equally shared. In compounds of nitrogen and oxygen, they “spend more time” slightly nearer the oxygen, with a higher electronegativity.
Confused? You should be. These are macroscopic explanations of quantum mechanical phenomena. But no matter. Let us look at a couple of rather common compounds of nitrogen and oxygen.
The simplest one, from its formula, is nitric oxide, NO. However, it is far from simple, since it is a free radical, meaning that the molecule has a single unpaired electron, making it extremely reactive. We chemists usually write its structural formula as N=O., with the equal sign representing a double bond (one sigma and one pi bond), and the dot representing the unpaired electron.
Molecules with a single unpaired electron are extremely reactive, for the most part. They do damage to living cells in many cases, and other mischief. But in the case of NO, things are a bit different. It is a neurotransmitter in mammals! Yes, this very highly reactive and potentially damaging compound actually serves to transmit nerve signals in certain systems. As a matter of fact, the drugs known as Viagra, Cilalis, and Levetra actually stimulate the release of NO in the corpus cavernosum of the human penis, and the NO causes the blood vessels that drain the penis to close, allowing for an erection. Those drugs also increase the NO levels elsewhere, and can cause severe cranial nerve damage (including temporary or more or less permanent loss of eyesight and/or hearing). Hmmmmm. Perhaps those TeeVee adverts should be looked at a little more in depth.
Now, NO is usually the culprit when oxygen and nitrogen are combined in a high temperature environment. But it also reacts at a diffusion controlled rate with atmospheric oxygen to form an extremely pernicious compound, nitrogen dioxide, NO2. This gas forms most of the brown look to smog that is still common in California, but much less that it would have been if air pollution laws had not been passed and enforced.
Nitrogen dioxide is extremely toxic, since the hydrolyzes to nitrous acid, and that gets further oxidized to nitric acid upon breathing it and thus exposing it to moisture in the lungs. And let us get one myth straightened out right here:
Nitrogen dioxide (along with its sister oxides, called NOx is produced in every internal combustion engine, even ones running on methane or hydrogen. NEVER be fooled to think that the only combustion product when using hydrogen as a fuel for cars is ONLY water. Nitrogen oxides are ALWAYS produced because of the high temperature reactions betwixt the fuel (in this case, pure hydrogen) and the oxidizer (in this case air, 78% nitrogen). There is ALWAYS a nitrogen oxide produced!
To limit the conversation a bit, it is important to realize that nitrogen dioxide exists in equilibrium with it dimer, dinitrogen tetroxide, N2O4. The monomer is the more highly colored of the two, so more brown looking at high temperatures where it is more stable. Both are about equally water reactive. That is one of many reasons that “smog” looks less dark when it is cold, because the monomer has reverted to the dimer, which has little color. It might still be harmful, if unseen. This is quite counterintuitive to me, because I would have expected the dimer to be more common at higher temperatures because of Le Chatelier’s principle. But experiment is more important than theory.
If we instead of adding an oxygen to NO but rather another nitrogen atom, we end up with nitrous oxide, N2O, also called laughing gas, the first medical anesthetic. It was discovered by the brilliant British chemist, Sir Humphrey Davy, and became quite popular early on as a recreational drug. Samuel Taylor Coleridge, who wrote The Rime of the Ancient Mariner, was a chum of Davy’s and they would have parties where this material was used. In his journals he recorded, and I have to paraphrase the following:
If there be a Heaven, its Atmosphere must be composed of this wonderful Gas.
I also strongly suspect that this is the origin of the term for something wonderful, “It’s a gas”. Nitrous oxide is still used extensively in dental practice (actually, the first use of it was the relatively painless removal of a tooth). I had a summer job working at a welding supply and cylinder gas supply company and I HATED delivering nitrous oxide. It turns out that, unlike oxygen or nitrogen, nitrous oxide exists as a liquid in the cylinder, and that makes them HEAVY! In addition to medical use, nitrous oxide is also used as an oxidizer for a couple of specialty applications, including flame atomic absorption spectroscopy (for metals quantitative analyses), and also in high performance automobile racing. Since nitrous oxide is a little over 33% oxygen compared to the atmosphere which is only 21% oxygen, fire burns faster (and thus hotter) when nitrous oxide is used. Pure oxygen is too dangerous for either of those applications due to the risk of explosion.
Ammonia is a compound of nitrogen and hydrogen, NH3, and an extremely important industrial chemical, since almost all other nitrogen compounds (except for the ones mined) are made from it. Most of us are familiar with household ammonia, a water solution of ammonia gas. It is an excellent cleaning agent, being basic enough to cut grease readily, but volatile so that it does not leave a residue. That is why it is often used in nonstreaking glass cleansing formulations. It is also used as a fertilizer, since nitrogen is one of the three most important plant nutrients.
Almost all ammonia produced today is from the Haber ammonia synthesis process, developed by Fritz Haber in Germany around the World War I era. Since nitrogen is an essential ingredient for almost all conventional (i.e., non nuclear) explosives, it was imperative that Germany develop a way to obtain it locally, since the vast nitrate deposits in South America were cut off during the war. In this process, nitrogen is produced from the air, and hydrogen from water, natural gas, or coal. The nitrogen and hydrogen are put in a pressure vessel, heated, and passed over a catalyst that lowers the activation energy for them to combine. Only a few per cent of the materials actually react, but since ammonia is easy to cool enough to be extracted as a liquid, and since nitrogen and hydrogen are not, the unreacted gases are recycled over and over again. The process is continuous, so new supplies of nitrogen and hydrogen are added to the reactor as needed.
This is a cheap and fairly efficient process for making ammonia, and from ammonia all of the important industrial nitrogen chemicals can be had, mostly by oxidation of the ammonia with the oxygen separated from the air that gave the pure nitrogen in the first place to our old friend nitrogen dioxide. That is reacted with water to form the extremely useful industrial chemical, nitric acid. Thus from only air and water, it is possible to produce some of the most useful materials in industry.
Nitric acid is used to make all sorts of things, from fertilizers to explosives to pharmaceuticals to plastics. It is absolutely for modern industry, and quite a hazardous material, even for a strong acid. It has a propensity to make anything made of cellulose extremely flammable, so fire and nitric acid sort of go hand in hand. Nitric acid is not available in very many consumer products, so is rarely seen by the public.
I mentioned earlier that amino acids, and hence proteins, all contain nitrogen, the word amino referring to an -NH2> group and acid, in organic chemistry, a -COOH group. Amino acids have the ability to link the amino group in one molecule with the acid group in a second (with the elimination of a water molecule) to form a peptide bond, the basic bond in proteins. Proteins can be thousands and thousands of amino acids long, and are the basic structural and enzymatic materials in all life.
Remember the Chinese material that went into dog food and killed the dogs? Since protein is high in nitrogen, a simple nitrogen analysis is often used to determine the price that a buyer is willing to pay for the material. Normally, the higher the nitrogen content, the higher the protein content, thus a product high in nitrogen is more valuable than a lower nitrogen content one. The Chinese adulterated the protein concentrate with melamine, a common and cheap industrial chemical with an extremely high nitrogen content. The analysis generally used to determine nitrogen is not sensitive to what kind of nitrogen it is, so the test did show high nitrogen, but was not able to tell that the nitrogen did not come from protein.
Nitrogen is also part of DNA and RNA, in the form of bases that are arranged on a scaffold of either deoxyribonucleic acid for DNA or just ribonucleic acid for RNA. DNA has the ability to make copies of itself because of hydrogen bonding betwixt two base pairs. This is important because if the two strands of DNA were chemically bonded to each other, they could not separate for replication. Since hydrogen bonds hold the two strands together, and hydrogen bonds only around 10% as strong as a “real” chemical bond, the strands can separate and new ones be created from each half strand.
Nitrogen is also present in the majority of pharmaceuticals in one form or another. For example, insulin is a protein and thus contains nitrogen as explained just above. Many pharmaceuticals are derived from alkaloids derived from plants, the nitrogen in an alkaloid giving them their alkaline character. Morphine, use to treat pain, is an alkaloid derived from the opium poppy. Cocaine (still used in medicine as a local anesthetic for eye surgery and for ear, nose, and throat surgery) is and alkaloid derived from the coca plant, originally from from South America. Penicillin also contains nitrogen.
Plants require large amounts of nitrogen to grow, because it is necessary for them to have to build proteins. Since nitrogen is only slowly replaced, modern agriculture depends on heavy use (too heavy, in many cases) of commercial fertilizer. (Phosphorous and potassium are also major components of commercial fertilizers). Most of this nitrogen is Haber process nitrogen, although to a limited extent some natural nitrates are mined for this use. Other than adding nitrogen (in a form that plants can use; they can not metabolize elemental nitrogen, so either ammonia, urea, or nitrates are added) by humans, the two major methods for replenishment of removed nitrogen include lightening and bacteria.
During thunderstorms, the high temperatures created by lightening cause the formation of small amounts of nitrogen dioxide, and this dissolves in rainwater to form nitric acid in very low concentration which falls on the soil. Basic minerals convert the acid to inorganic nitrates, which plants can use. Obviously, this is a very slow process.
Bacteria are faster. Some plants, particularly many legumes (members of the pea family) harbor bacteria in nodules on their roots that do have the ability to convert atmospheric nitrogen to usable forms, most often ammonia. Interestingly, many of these plants also produce seeds that are high in protein, and hence nitrogen. Common examples include many kinds of beans, especially soybeans. By rotating crops, planting soybeans one year and, say, corn (which does NOT contribute nitrogen) it is possible to keep a reasonable amount of nitrogen in the soil without the overuse of chemical fertilizers.
I hope that this brief introduction to this extremely important chemical element has been interesting and informative. We shall now present another installment of Ed Lawrence’s recollections about being on the development team for the 8-Track system, continued from last week. I really appreciate Ed writing this, specifically for Pique the Geek readers.
Back to the time line. There were other people I worked with or near as well. Bob Wells and Darrell Zielke worked in the room next to us. I think their jobs dealt with various avionics for the Lear Jet. They had a temperature chamber that we were allowed to use when necessary.
Bob was a tall man and never said too much. He seemed either very controlled or a bit morose. I never could figure him out, as he was a very private person.
Darrell was an entirely different sort. Happy, handsome fellow that he was, he had married one of the most beautiful women I have ever met. I believe Darrell was also a Amateur Radio Operator and presently lives in the state of Washington. Because of his always-reasoned outlook on life, Darrell is high on the short list of people I am glad to have known.
One other interesting fellow was Lee Littlefield. Blessed with a dry sense of humor, he was a bit somber in appearance, looking something along the lines of a thin Humphrey Bogart. Lee related the following event to me. He said that he had only been employed a couple of weeks, and was hard at work, bent over a drawing at his desk. Lee never wore a tie as they made him uncomfortable. A gruff voice from behind him said, “Why are you not wearing a tie?” Without looking up from his work, Lee replied, “‘Cause I came here to work, not to look pretty!” Silence followed. After half a minute or so, Lee realized that he perhaps should know who had spoken to him. He looked up and saw Bill Lear about to leave the area; apparently satisfied with the answer he had received. Lee became one of Lear’s preferred engineers for difficult problems.
I participated in an interesting experiment. An influential customer complained that he could hear a pitch change in the music from his 8-track when he turned a sharp corner. Bill wanted this investigated, and I was chosen to do the testing. I was to drive Bill Lear’s Caddy out on an empty area of the airport apron usually reserved for aircraft and service vehicles. My feeling was that the customer imagined the pitch change as the mathematics indicated it would not be discernable to human senses. With Frank Hacker acting as the observer, I proceeded to put Bill Lear’s Caddy through a series of sharp turns in either direction, at higher turn rates. I didn’t quite get the Caddy up on two wheels, but I came close! Poor Frank! He stayed glued up against the passenger’s door, bug-eyed with fright like a nervous virgin at a drive-in movie with a sex-fiend! I’m certain that any of the security guards thought two nuts had somehow stolen Lear’s car keys, and were out to destroy the world! The tests verified that there was no change in the sound, even with violent turns. This was reported back to Mr. Lear, and the matter was closed. It was a lot of fun, though!
One more item I am certain is not widely known: It was late one evening when we got the first of the production prototypes assembled and functioning as designed. The head salesman was there to witness this, although it was about midnight. He thought that this called for a drink, but the only booze in the place was in Bill Lear’s office. He had an office key but not one to the liquor cabinet.
I said, ” I can get in there.” (I was well known for my ability to procure needed parts held in locked places.) The salesman looked startled. “You can’t damage Bill’s liquor cabinet!” I assured that I would do no damage in the process. I had some inside information. A few days earlier, I had been tasked with installing a recording device for Lear’s private office use, and had seen the cabinet opened in my presence. I had observed the nature of the locking device, and knew how easy it would be to defeat it. We went down to the office, and I opened the liquor cabinet more quickly than it could have been opened with the key. I suspect I am the only man it the world who ever broke into Bill Lear’s “private stock” and got away with it, clean! I did not have a sample myself, as I am a non-drinker.Things finally began working well together. The testing was nearing completion on various models, especially on the one with the AM radio in addition to the 8-track player. Bugs had mostly been worked out of all sub-systems, including the tape cartridge. Only ramp-up to full production remained, and that required a move to larger quarters: a whole new plant!
One of the unusual aspects of working for Lear was that a few of the customers for the Lear Jet were very prominent people. It is quite widely known that his private bodyguards always attended Frank Sinatra. We got word that “The Voice” was coming to inspect the progress on his personal LearJet. The excitement level in the plant rose to unprecedented levels as the expected arrival time neared. No official word was passed that we should not bother Frank, or even greet him in any way. It was not needed. We knew that Bill Lear would not look favorably on anything less than pure professionalism on our part. Remember the bit about the tie? We worked at our tasks as normal. Then suddenly, Bill Lear appeared, escorting Frank into our little lab, no bodyguard in sight. I was standing about 12 feet from “Old Blue Eyes”, he looking neat and dapper, his right hand in his pocket, the little scar on his right cheek as plain as day. One quick look, and I returned to my work. No one spoke a word nor paid any attention to this giant celebrity in our room. Unasked, we gave Bill Lear what we knew he wanted.
Later, in Detroit, another such celebrity toured our plant. That was Danny Kaye. He looked just like his pictures. These two were the ones I personally observed. I did not track the others who came through, but there were more than a few.
The new 8-track unit was almost production ready. It was important to locate our manufacturing facility close to the major market consumers. This meant being close to Detroit. It was impractical to take everyone, and many of the affected employees would have been unable to take a position in Michigan even if it were offered. The core knowledge was in the Engineering and Management Departments. About 21 of us were selected to relocate to the Detroit area.
The rumor was that Novi, MI was the selected site, about 30 miles northwest of Detroit. When the details were finally released, it turned out to be in one of the northern Detroit suburbs. (I don’t remember exactly where, but I suspect that I could drive there if there was a need.)
Well, you have done it again! You have wasted many einsteins of perfectly good photons reading this airy piece. And even though Mike Huckabee decries the use of torture when he reads me say it, I always learn much more than I could ever hope to teach writing this series, so keep those comments, questions, corrections, and other feedback coming. Tips and recs are also very welcome. Remember, no science or technology issue is off topic here. I shall stick around as long as comments warrant, and shall return tomorrow evening around the same time for stragglers.
Happy Mothers’ Day, AND remember that 66 years ago today the Allies declared victory in Europe for World War II.
Warmest regards,
Doc

1 comments
Author
some basic chemistry?
Warmest regards,
Doc