Last time we started our series on carbon, and I now expect it to run for four installments. Amongst many other properties, carbon is unique in having more allotropic forms than any other element. Also known as allotropes, these are pure elements with radically different properties. The term is reserved only for elements, the term for compounds being polymorphs. An allotrope is a subset of a polymorph.
There is also another distinction: for an element to have an allotrope, it must exist in the same phase. Thus, solid lead and molten lead (and lead vapor) are not allotropes, but rather different phases of a given element.
Before we concentrate on carbon, let us consider oxygen. In the gaseous phase, it has two allotropes, O2, normal molecular oxygen, and O3, also called ozone. For an element as reactive as oxygen, normal molecular oxygen is remarkably nonreactive (wait a few weeks), but ozone is extremely reactive. But both are just composed of oxygen atoms.
Carbon is sort of unusual in that it is an element that we can see in a fairly pure form in normal life. Most pure elements are either gases (think nitrogen or oxygen) or sort of unusual, like pure gold (almost never seen pure), silver (also almost never seen pure), or compounds of them with other elements. Copper is an exception, because it has to be very pure to have the optimum electrical conductivity required for power transmission, so most of us have seen pure copper.
Most all of us are familiar with the three most common allotropes of carbon: graphite, diamond, and amorphous carbon. But there are more! Graphine, the fullerines, and others are known, and there are some not yet observed that theory predict. Let us examine materials that consist of only pure, or as pure as feasible, carbon.
Just about everyone has seen and handled graphite. It is the stuff of pencil “leads” (before graphite was used in pencils, lead was actually used, hence the term lead). Interestingly, an old name for natural graphite is plumbago, from the Latin plumbum, meaning lead. One reason that makes it good for a pencil lead is that it is really soft. It is so soft that it is usually ground up and mixed with a binder, either clay or synthetic polymers, and baked to harden it. The hardness rating of pencils depends on how much binder is used, the harder ones having more binder. Graphite is also useful for pencils because it is nontoxic, water and solvent proof, and very dark. Even though it is great for pencil leads, that use accounts for only about 4% of US consumption.
So why is graphite so soft? It has to do with its physical structure, which in turn has to do with its electronic structure. Here is a picture of the crystal structure of graphite. You will notice that graphite is composed of layers of carbon atoms bonded to each other.
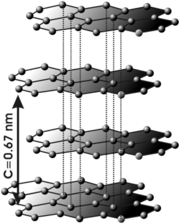
In a given layer, each carbon atom is bonded to three more carbon atoms (except at the edges, and in real graphite the sheets are much wider and longer than in the picture) forming six membered rings. These bonds are sp2 hybrids, very strong in themselves, but are also aromatic, giving graphite extreme stability. Betwixt layers there are no true bonds, but only very weak van der Waals interactions. These interactions are technically called induced dipole/induced dipole interactions and are caused by random shifts in electron density in regions betwixt the layers. As I said, they are very weak indeed.
So we have an extremely strong and stable layer of a “supermolecule” weakly attracted to another such layer. As the pencil is rubbed across the paper, the layers separate and are attracted by van der Waals forces to the paper, although there is also a lot of simple mechanical entrainment of the graphite molecules in nooks and crannies in the paper. This is why it is easy to erase pencil marks, because the forces holding the graphite to the paper are week indeed. In addition, the eraser removes a tiny amount of the paper carrying the graphite.
This structure accounts for all of the properties of graphite. Except for defects and aberrations along the edges of the sp2 structure, it is a perfect aromatic compound. Furthermore, the electrons bonding the whole thing together are highly delocalized, meaning that graphite, at least along the layers of the aromatic structure, conducts electricity fairly well, and extremely well for a nonmetal. The ramifications of that are enormous.
Most people know that graphite is one of the very best lubricants, especially for high temperature use. It is also preferred in locks and other delicate mechanisms because it does not evaporate nor polymerize and leave a gummy residue. One reason that was thought to be responsible is the layers of aromatic structure slide past each other, and that probably is partially responsible. However, graphite has practically no lubricity in a vacuum, so there is more going on than slippage planes. Evidently there is some interaction betwixt the graphite and fluids (graphite also has lubricity when wetted with liquids) that provide lubricity. Lubricants are another small market for graphite. By the way, graphite attacks aluminum and should not be used to lubricate objects made of it.
Graphite is very anisotropic, meaning that its properties, particularly those of thermal and electrical conductivity, depend on the direction of propagation. This makes sense because of the supermolecule nature of the layers and the very weak interaction betwixt layers. Thus, heat and electricity readily pass along the layers but hardly at all betwixt them.
By far the largest use of graphite is as a refractory material, a material that can withstand extreme temperatures. The largest use of graphite is in as refractories, and this is actually a very old use for it. As far back as Elizabethan times graphite was used to line molds for cannonballs, and it is still unrivaled for certain applications.
It is used in steelmaking both as a refractory liner for blast furnaces and to add carbon to get the desired level in finished steel. These are huge uses. It is used in aluminum refining as the both the cathode (positive electrode) and the anode (negative electrode). At the anode oxygen is formed and the anode is burnt away, so the anodes are rigged in such a way that they can be lowered into the reactor as they are consumed. The cathodes last a lot longer, but finally wear out and have to be replaced, but that is on the order of years.
Lots of graphite is also used as linings for brakes for large vehicles. Those generate a lot of heat and graphite can take it. It is always mixed with other materials because it is a lubricant. Generally it is used in the form of reinforced carbon-carbon, where graphite fibre cloth is coated with an organic material like pitch with added carbon in the binder. Then it is molded and heated, then finally finished by passing acetylene gas through it at high temperature to fill voids and grow large crystals of graphite. This material is also used for aerospace applications for areas of spacecraft (like the space shuttle wing leading edges and tips) that will be exposed to extreme heat during reentry.
A growing use of graphite is in batteries. This is not new, as from the beginning dry cells used a graphite anode. However, lithium ion batteries require a highly purified graphite for their anodes, and with the digital revolution lithium ion batteries are coming on strong. Electric vehicles that use these batteries are expected to be a huge market in the coming years.
Most of these applications can use either natural or synthetic graphite. The higher tech ones tend to use synthetic graphite because its properties can be controlled more closely, so lithium ion battery graphite is usually synthetic. It costs a lot of electricity to make graphite. Typical power requirements are somewhere around ten megawatts, but the graphite is quite pure.
This just scratches the surface of the uses of graphite, to coin a term. Now we shall look at another allotrope of carbon that almost everyone has seen and touched, diamond.
For two things to be made of nothing but carbon (with some trace impurities), diamond and graphite could not be more different. Whilst graphite is one of the softest materials known, diamond (specifically laboratory produced diamond, the crystals of some of which approach perfection) is the hardest material known. Graphite is always opaque and black, where high quality diamonds are water white and clear. Graphite is only a fair conductor of heat, whilst diamond has the highest thermal conductivity of any known substance, 7.5 times greater than copper! Why these differences? We have to look at the physical, and hence, the electronic structure of diamond and contrast them with graphite.
Here is a nice video showing the structures of diamond and graphite.
Note that in diamond, except at the edges, each carbon is connected to four other carbons rather than only three. Since carbon requires four bonds, in diamond all of the bonds are single bonds. There is no aromatic character in diamond, so it is a different kind of supermolecule than is graphite. Who says that molecules are too small to see? A diamond is a single molecule!
Not having aromatic character has huge implications. First, diamonds do not conduct electricity because all of the electrons are localized. Second, they do not absorb light for the same reason. Carbon-carbon single bonds are pretty strong, so diamonds are chemically inert for the most part and because there are not layers like in graphite, diamond is rigid and hard. Because of the rigidity of the bonds, diamond conducts vibrations readily, that this is the cause for its extremely high thermal conductivity.
Aside for gem and investment uses where the beauty, clarity, and large size are important, the most common industrial use for diamonds is as abrasives because of the extreme hardness. Interestingly, natural diamonds vary in hardness but even the softest diamonds are harder than most other materials. Actually, there are a lot of diamonds mined (around 135,000,000 carets, or 27,000 kg), but only 20% of those are gem quality. Most of the rest go for abrasives. That is not nearly enough to meet demand, so another 110,000 kg are produced artificially for abrasives.
Folks, we are going to have to cut it here. My computer is acting funky and it is close to posting time. We shall take up where we left off next time. I apologize for not finishing the piece, but quite honestly I have been ill with a cold since Thursday and have not had a lot of energy to write. I missed Popular Culture Friday for that reason.
I shall leave out the traditional joke tonight and just say that I will stay here for as long as comments warrant tonight, and please provide questions, comments, corrections and other feedback. Tips and recs are also always welcome. Remember, no science or technology issue is off topic in the comments. I shall return tomorrow evening for Review Time as well.
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at
Docudharma, and

4 comments
Skip to comment form
Author
how different things can be?
Warmest regards,
Doc